Identification of the Binding Partners for Hspb2 and Cryab Reveals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genome-Wide Association Study of Increasing Suicidal Ideation During Antidepressant Treatment in the GENDEP Project
The Pharmacogenomics Journal (2012) 12, 68–77 & 2012 Macmillan Publishers Limited. All rights reserved 1470-269X/12 www.nature.com/tpj ORIGINAL ARTICLE Genome-wide association study of increasing suicidal ideation during antidepressant treatment in the GENDEP project NPerroud1,2,3,RUher1, Suicidal thoughts during antidepressant treatment have been the focus of 1 2,3,4 several candidate gene association studies. The aim of the present genome- MYM Ng , M Guipponi , wide association study was to identify additional genetic variants involved in 5 6 JHauser, N Henigsberg , increasing suicidal ideation during escitalopram and nortriptyline treatment. WMaier7,OMors8, A total of 706 adult participants of European ancestry, treated for major M Gennarelli9, M Rietschel10, depression with escitalopram or nortriptyline over 12 weeks in the Genome- DSouery11, MZ Dernovsek12, Based Therapeutic Drugs for Depression (GENDEP) study were genotyped 13 14 with Illumina Human 610-Quad Beadchips (Illumina, San Diego, CA, USA). AS Stamp ,MLathrop , A total of 244 subjects experienced an increase in suicidal ideation AFarmer1, G Breen1,KJAitchison1, during follow-up. The genetic marker most significantly associated with CM Lewis1,IWCraig1 increasing suicidality (8.28 Â 10À7) was a single-nucleotide polymorphism and P McGuffin1 (SNP; rs11143230) located 30 kb downstream of a gene encoding guanine deaminase (GDA) on chromosome 9q21.13. Two suggestive drug-specific 1MRC Social, Genetic and Developmental Psychiatry associations within KCNIP4 (Kv channel-interacting protein 4; chromosome Centre, Institute of Psychiatry, King’s College 4p15.31) and near ELP3 (elongation protein 3 homolog; chromosome London, London, UK; 2Department of Psychiatry, 8p21.1) were found in subjects treated with escitalopram. -

Transcriptional Regulation of RKIP in Prostate Cancer Progression
Health Science Campus FINAL APPROVAL OF DISSERTATION Doctor of Philosophy in Biomedical Sciences Transcriptional Regulation of RKIP in Prostate Cancer Progression Submitted by: Sandra Marie Beach In partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biomedical Sciences Examination Committee Major Advisor: Kam Yeung, Ph.D. Academic William Maltese, Ph.D. Advisory Committee: Sonia Najjar, Ph.D. Han-Fei Ding, M.D., Ph.D. Manohar Ratnam, Ph.D. Senior Associate Dean College of Graduate Studies Michael S. Bisesi, Ph.D. Date of Defense: May 16, 2007 Transcriptional Regulation of RKIP in Prostate Cancer Progression Sandra Beach University of Toledo ACKNOWLDEGMENTS I thank my major advisor, Dr. Kam Yeung, for the opportunity to pursue my degree in his laboratory. I am also indebted to my advisory committee members past and present, Drs. Sonia Najjar, Han-Fei Ding, Manohar Ratnam, James Trempe, and Douglas Pittman for generously and judiciously guiding my studies and sharing reagents and equipment. I owe extended thanks to Dr. William Maltese as a committee member and chairman of my department for supporting my degree progress. The entire Department of Biochemistry and Cancer Biology has been most kind and helpful to me. Drs. Roy Collaco and Hong-Juan Cui have shared their excellent technical and practical advice with me throughout my studies. I thank members of the Yeung laboratory, Dr. Sungdae Park, Hui Hui Tang, Miranda Yeung for their support and collegiality. The data mining studies herein would not have been possible without the helpful advice of Dr. Robert Trumbly. I am also grateful for the exceptional assistance and shared microarray data of Dr. -

Protein Interaction Network of Alternatively Spliced Isoforms from Brain Links Genetic Risk Factors for Autism
ARTICLE Received 24 Aug 2013 | Accepted 14 Mar 2014 | Published 11 Apr 2014 DOI: 10.1038/ncomms4650 OPEN Protein interaction network of alternatively spliced isoforms from brain links genetic risk factors for autism Roser Corominas1,*, Xinping Yang2,3,*, Guan Ning Lin1,*, Shuli Kang1,*, Yun Shen2,3, Lila Ghamsari2,3,w, Martin Broly2,3, Maria Rodriguez2,3, Stanley Tam2,3, Shelly A. Trigg2,3,w, Changyu Fan2,3, Song Yi2,3, Murat Tasan4, Irma Lemmens5, Xingyan Kuang6, Nan Zhao6, Dheeraj Malhotra7, Jacob J. Michaelson7,w, Vladimir Vacic8, Michael A. Calderwood2,3, Frederick P. Roth2,3,4, Jan Tavernier5, Steve Horvath9, Kourosh Salehi-Ashtiani2,3,w, Dmitry Korkin6, Jonathan Sebat7, David E. Hill2,3, Tong Hao2,3, Marc Vidal2,3 & Lilia M. Iakoucheva1 Increased risk for autism spectrum disorders (ASD) is attributed to hundreds of genetic loci. The convergence of ASD variants have been investigated using various approaches, including protein interactions extracted from the published literature. However, these datasets are frequently incomplete, carry biases and are limited to interactions of a single splicing isoform, which may not be expressed in the disease-relevant tissue. Here we introduce a new interactome mapping approach by experimentally identifying interactions between brain-expressed alternatively spliced variants of ASD risk factors. The Autism Spliceform Interaction Network reveals that almost half of the detected interactions and about 30% of the newly identified interacting partners represent contribution from splicing variants, emphasizing the importance of isoform networks. Isoform interactions greatly contribute to establishing direct physical connections between proteins from the de novo autism CNVs. Our findings demonstrate the critical role of spliceform networks for translating genetic knowledge into a better understanding of human diseases. -
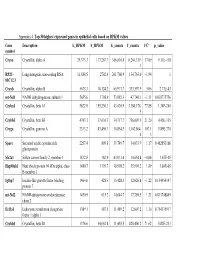
Appendix 4. Top 50 Highest Expressed Genes in Epithelial Cells Based on RPKM Values
Appendix 4. Top 50 highest expressed genes in epithelial cells based on RPKM values Gene Description E_RPKM F_RPKM E_counts F_counts FC* p_value symbol Cryaa Crystallin, alpha A 29,373.3 177,267.7 366,616.4 6,264,319. 17.09 9.11E-118 1 RP23– Long intergenic non-coding RNA 11,888.5 2702.4 261,760.9 134,763.0 −1.94 1 81C12.3 Cryab Crystallin, alpha B 5673.3 10,124.2 65,971.7 333,597.9 5.06 2.71E-43 mt-Nd1 NADH dehydrogenase, subunit 1 5655.6 1798.9 53,082.3 47,748.1 −1.11 0.838775756 Cryba1 Crystallin, beta A1 5622.0 155,230.3 43,420.9 3,380,176. 77.85 1.34E-240 5 Crybb3 Crystallin, beta B3 4743.1 37,636.3 34,717.7 736,007.9 21.20 4.45E-135 Cryga Crystallin, gamma A 2333.2 83,496.3 10,854.5 1,162,864. 107.1 5.89E-270 6 3 Sparc Secreted acidic cysteine rich 2257.4 809.8 39,749.7 34,033.9 −1.17 0.462853166 glycoprotein Slc2a1 Solute carrier family 2, member 1 1832.8 162.9 43,031.4 10,654.8 −4.04 1.67E-05 Hsp90ab1 Heat shock protein 90 kDa alpha, class 1480.7 1139.7 18,998.2 35,901.2 1.89 3.84E-05 B member 1 Igfbp7 Insulin-like growth factor binding 1464.6 428.3 15,428.3 12,626.8 −1.22 0.154954147 protein 7 mt-Nd2 NADH-ubiquinone oxidoreductase 1450.9 615.2 14,644.7 17,789.5 1.21 0.833748849 chain 2 Eef1a1 Eukaryotic translation elongation 1389.1 587.5 11,489.2 12,607.2 1.10 0.754135917 factor 1 alpha 1 Crybb1 Crystallin, beta B1 1376.6 34,662.8 11,455.5 820,406.2 71.62 5.82E-233 Htra3 HtrA serine peptidase 3 1338.6 162.0 23,197.6 6433.9 −3.61 3.93E-05 Gnb2l1 Guanine nucleotide-binding protein 1293.3 670.1 14,495.1 21,652.1 1.49 0.001685952 -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Views of the NIH
CLINICAL EPIDEMIOLOGY www.jasn.org Genetic Variants Associated with Circulating Fibroblast Growth Factor 23 Cassianne Robinson-Cohen ,1 Traci M. Bartz,2 Dongbing Lai,3 T. Alp Ikizler,1 Munro Peacock,4 Erik A. Imel,4 Erin D. Michos,5 Tatiana M. Foroud,3 Kristina Akesson,6,7 Kent D. Taylor,8 Linnea Malmgren,6,7 Kunihiro Matsushita,5,9,10 Maria Nethander,11 Joel Eriksson,12 Claes Ohlsson,12 Daniel Mellström,12 Myles Wolf,13 Osten Ljunggren,14 Fiona McGuigan,6,7 Jerome I. Rotter,8 Magnus Karlsson,6,7 Michael J. Econs,3,4 Joachim H. Ix,15,16 Pamela L. Lutsey,17 Bruce M. Psaty,18,19 Ian H. de Boer ,20 and Bryan R. Kestenbaum 20 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background Fibroblast growth factor 23 (FGF23), a bone-derived hormone that regulates phosphorus and vitamin D metabolism, contributes to the pathogenesis of mineral and bone disorders in CKD and is an emerging cardiovascular risk factor. Central elements of FGF23 regulation remain incompletely under- stood; genetic variation may help explain interindividual differences. Methods We performed a meta-analysis of genome-wide association studies of circulating FGF23 con- centrations among 16,624 participants of European ancestry from seven cohort studies, excluding par- ticipants with eGFR,30 ml/min per 1.73 m2 to focus on FGF23 under normal conditions. We evaluated the association of single-nucleotide polymorphisms (SNPs) with natural log–transformed FGF23 concentra- tion, adjusted for age, sex, study site, and principal components of ancestry. -

The Inactive X Chromosome Is Epigenetically Unstable and Transcriptionally Labile in Breast Cancer
Supplemental Information The inactive X chromosome is epigenetically unstable and transcriptionally labile in breast cancer Ronan Chaligné1,2,3,8, Tatiana Popova1,4, Marco-Antonio Mendoza-Parra5, Mohamed-Ashick M. Saleem5 , David Gentien1,6, Kristen Ban1,2,3,8, Tristan Piolot1,7, Olivier Leroy1,7, Odette Mariani6, Hinrich Gronemeyer*5, Anne Vincent-Salomon*1,4,6,8, Marc-Henri Stern*1,4,6 and Edith Heard*1,2,3,8 Extended Experimental Procedures Cell Culture Human Mammary Epithelial Cells (HMEC, Invitrogen) were grown in serum-free medium (HuMEC, Invitrogen). WI- 38, ZR-75-1, SK-BR-3 and MDA-MB-436 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS). DNA Methylation analysis. We bisulfite-treated 2 µg of genomic DNA using Epitect bisulfite kit (Qiagen). Bisulfite converted DNA was amplified with bisulfite primers listed in Table S3. All primers incorporated a T7 promoter tag, and PCR conditions are available upon request. We analyzed PCR products by MALDI-TOF mass spectrometry after in vitro transcription and specific cleavage (EpiTYPER by Sequenom®). For each amplicon, we analyzed two independent DNA samples and several CG sites in the CpG Island. Design of primers and selection of best promoter region to assess (approx. 500 bp) were done by a combination of UCSC Genome Browser (http://genome.ucsc.edu) and MethPrimer (http://www.urogene.org). All the primers used are listed (Table S3). NB: MAGEC2 CpG analysis have been done with a combination of two CpG island identified in the gene core. Analysis of RNA allelic expression profiles (based on Human SNP Array 6.0) DNA and RNA hybridizations were normalized by Genotyping console. -

A Gene Expression Resource Generated by Genome-Wide Lacz
© 2015. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2015) 8, 1467-1478 doi:10.1242/dmm.021238 RESOURCE ARTICLE A gene expression resource generated by genome-wide lacZ profiling in the mouse Elizabeth Tuck1,**, Jeanne Estabel1,*,**, Anika Oellrich1, Anna Karin Maguire1, Hibret A. Adissu2, Luke Souter1, Emma Siragher1, Charlotte Lillistone1, Angela L. Green1, Hannah Wardle-Jones1, Damian M. Carragher1,‡, Natasha A. Karp1, Damian Smedley1, Niels C. Adams1,§, Sanger Institute Mouse Genetics Project1,‡‡, James N. Bussell1, David J. Adams1, Ramiro Ramırez-Soliś 1, Karen P. Steel1,¶, Antonella Galli1 and Jacqueline K. White1,§§ ABSTRACT composite of RNA-based expression data sets. Strong agreement was observed, indicating a high degree of specificity in our data. Knowledge of the expression profile of a gene is a critical piece of Furthermore, there were 1207 observations of expression of a information required to build an understanding of the normal and particular gene in an anatomical structure where Bgee had no essential functions of that gene and any role it may play in the data, indicating a large amount of novelty in our data set. development or progression of disease. High-throughput, large- Examples of expression data corroborating and extending scale efforts are on-going internationally to characterise reporter- genotype-phenotype associations and supporting disease gene tagged knockout mouse lines. As part of that effort, we report an candidacy are presented to demonstrate the potential of this open access adult mouse expression resource, in which the powerful resource. expression profile of 424 genes has been assessed in up to 47 different organs, tissues and sub-structures using a lacZ reporter KEY WORDS: Gene expression, lacZ reporter, Mouse, Resource gene. -

A Comprehensive Analysis of the Expression of Crystallins in Mouse Retina Jinghua Xi Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2003 A comprehensive analysis of the expression of crystallins in mouse retina Jinghua Xi Washington University School of Medicine in St. Louis Rafal Farjo University of Michigan - Ann Arbor Shigeo Yoshida University of Michigan - Ann Arbor Timothy S. Kern Case Western Reserve University Anand Swaroop University of Michigan - Ann Arbor See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Xi, Jinghua; Farjo, Rafal; Yoshida, Shigeo; Kern, Timothy S.; Swaroop, Anand; and Andley, Usha P., ,"A comprehensive analysis of the expression of crystallins in mouse retina." Molecular Vision.9,. 410-419. (2003). https://digitalcommons.wustl.edu/open_access_pubs/1801 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Authors Jinghua Xi, Rafal Farjo, Shigeo Yoshida, Timothy S. Kern, Anand Swaroop, and Usha P. Andley This open access publication is available at Digital Commons@Becker: https://digitalcommons.wustl.edu/open_access_pubs/1801 Molecular Vision 2003; 9:410-9 <http://www.molvis.org/molvis/v9/a53> © 2003 Molecular Vision Received 28 May 2003 | Accepted 19 August 2003 | Published 28 August 2003 A comprehensive analysis of the expression of crystallins in mouse retina Jinghua Xi,1 Rafal Farjo,3 Shigeo Yoshida,3 Timothy S. Kern,5 Anand Swaroop,3,4 Usha P. Andley1,2 Departments of 1Ophthalmology and Visual Sciences and 2Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. -

A Dominant Mutation in RPE65 Identified by Whole-Exome
A Dominant Mutation in RPE65 Identified by Whole-Exome Sequencing Causes Retinitis Pigmentosa with Choroidal Involvement Peter Humphries, Sara J Bowne, Marian M Humphries, Lori S Sullivan, Paul F Kenna, Lawrence Cs Tam, Anna-Sophia Kiang, Matthew Campbell, George M Weinstock, Daniel C Koboldt, et al. To cite this version: Peter Humphries, Sara J Bowne, Marian M Humphries, Lori S Sullivan, Paul F Kenna, et al.. A Dominant Mutation in RPE65 Identified by Whole-Exome Sequencing Causes Retinitis Pigmentosa with Choroidal Involvement. European Journal of Human Genetics, Nature Publishing Group, 2011, 10.1038/ejhg.2011.86. hal-00649449 HAL Id: hal-00649449 https://hal.archives-ouvertes.fr/hal-00649449 Submitted on 8 Dec 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Edited manuscript for European Journal of Human Genetics April 4th 2011. “A Dominant Mutation in RPE65 Identified by Whole-Exome Sequencing Causes Retinitis Pigmentosa with Choroidal Involvement” Sara J. Bowne1*, Marian M. Humphries2*, Lori S. Sullivan1*, Paul F. Kenna2,3*, Lawrence C.S. Tam2, Anna Sophia Kiang2, Matthew Campbell2, George M. Weinstock4, Daniel C. Koboldt4, Li Ding4, Robert S. Fulton4, Erica J. Sodergren4, Denis Alman2, Sophia Millington-Ward2, Arpad Palfi2, Alex McKee2, Susan H. -

Supplemental Information
Supplemental information Dissection of the genomic structure of the miR-183/96/182 gene. Previously, we showed that the miR-183/96/182 cluster is an intergenic miRNA cluster, located in a ~60-kb interval between the genes encoding nuclear respiratory factor-1 (Nrf1) and ubiquitin-conjugating enzyme E2H (Ube2h) on mouse chr6qA3.3 (1). To start to uncover the genomic structure of the miR- 183/96/182 gene, we first studied genomic features around miR-183/96/182 in the UCSC genome browser (http://genome.UCSC.edu/), and identified two CpG islands 3.4-6.5 kb 5’ of pre-miR-183, the most 5’ miRNA of the cluster (Fig. 1A; Fig. S1 and Seq. S1). A cDNA clone, AK044220, located at 3.2-4.6 kb 5’ to pre-miR-183, encompasses the second CpG island (Fig. 1A; Fig. S1). We hypothesized that this cDNA clone was derived from 5’ exon(s) of the primary transcript of the miR-183/96/182 gene, as CpG islands are often associated with promoters (2). Supporting this hypothesis, multiple expressed sequences detected by gene-trap clones, including clone D016D06 (3, 4), were co-localized with the cDNA clone AK044220 (Fig. 1A; Fig. S1). Clone D016D06, deposited by the German GeneTrap Consortium (GGTC) (http://tikus.gsf.de) (3, 4), was derived from insertion of a retroviral construct, rFlpROSAβgeo in 129S2 ES cells (Fig. 1A and C). The rFlpROSAβgeo construct carries a promoterless reporter gene, the β−geo cassette - an in-frame fusion of the β-galactosidase and neomycin resistance (Neor) gene (5), with a splicing acceptor (SA) immediately upstream, and a polyA signal downstream of the β−geo cassette (Fig. -

RET Gene Fusions in Malignancies of the Thyroid and Other Tissues
G C A T T A C G G C A T genes Review RET Gene Fusions in Malignancies of the Thyroid and Other Tissues Massimo Santoro 1,*, Marialuisa Moccia 1, Giorgia Federico 1 and Francesca Carlomagno 1,2 1 Department of Molecular Medicine and Medical Biotechnology, University of Naples “Federico II”, 80131 Naples, Italy; [email protected] (M.M.); [email protected] (G.F.); [email protected] (F.C.) 2 Institute of Endocrinology and Experimental Oncology of the CNR, 80131 Naples, Italy * Correspondence: [email protected] Received: 10 March 2020; Accepted: 12 April 2020; Published: 15 April 2020 Abstract: Following the identification of the BCR-ABL1 (Breakpoint Cluster Region-ABelson murine Leukemia) fusion in chronic myelogenous leukemia, gene fusions generating chimeric oncoproteins have been recognized as common genomic structural variations in human malignancies. This is, in particular, a frequent mechanism in the oncogenic conversion of protein kinases. Gene fusion was the first mechanism identified for the oncogenic activation of the receptor tyrosine kinase RET (REarranged during Transfection), initially discovered in papillary thyroid carcinoma (PTC). More recently, the advent of highly sensitive massive parallel (next generation sequencing, NGS) sequencing of tumor DNA or cell-free (cfDNA) circulating tumor DNA, allowed for the detection of RET fusions in many other solid and hematopoietic malignancies. This review summarizes the role of RET fusions in the pathogenesis of human cancer. Keywords: kinase; tyrosine kinase inhibitor; targeted therapy; thyroid cancer 1. The RET Receptor RET (REarranged during Transfection) was initially isolated as a rearranged oncoprotein upon the transfection of a human lymphoma DNA [1].