University of California, San Diego
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
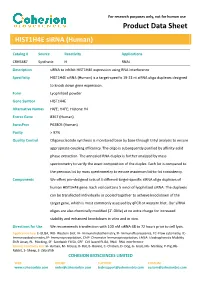
Product Data Sheet
For research purposes only, not for human use Product Data Sheet HIST1H4E siRNA (Human) Catalog # Source Reactivity Applications CRH5487 Synthetic H RNAi Description siRNA to inhibit HIST1H4E expression using RNA interference Specificity HIST1H4E siRNA (Human) is a target-specific 19-23 nt siRNA oligo duplexes designed to knock down gene expression. Form Lyophilized powder Gene Symbol HIST1H4E Alternative Names H4/E; H4FE; Histone H4 Entrez Gene 8367 (Human) SwissProt P62805 (Human) Purity > 97% Quality Control Oligonucleotide synthesis is monitored base by base through trityl analysis to ensure appropriate coupling efficiency. The oligo is subsequently purified by affinity-solid phase extraction. The annealed RNA duplex is further analyzed by mass spectrometry to verify the exact composition of the duplex. Each lot is compared to the previous lot by mass spectrometry to ensure maximum lot-to-lot consistency. Components We offers pre-designed sets of 3 different target-specific siRNA oligo duplexes of human HIST1H4E gene. Each vial contains 5 nmol of lyophilized siRNA. The duplexes can be transfected individually or pooled together to achieve knockdown of the target gene, which is most commonly assessed by qPCR or western blot. Our siRNA oligos are also chemically modified (2’-OMe) at no extra charge for increased stability and enhanced knockdown in vitro and in vivo. Directions for Use We recommends transfection with 100 nM siRNA 48 to 72 hours prior to cell lysis. Application key: E- ELISA, WB- Western blot, IH- Immunohistochemistry, -

University of California, San Diego
UNIVERSITY OF CALIFORNIA, SAN DIEGO The post-terminal differentiation fate of RNAs revealed by next-generation sequencing A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Biomedical Sciences by Gloria Kuo Lefkowitz Committee in Charge: Professor Benjamin D. Yu, Chair Professor Richard Gallo Professor Bruce A. Hamilton Professor Miles F. Wilkinson Professor Eugene Yeo 2012 Copyright Gloria Kuo Lefkowitz, 2012 All rights reserved. The Dissertation of Gloria Kuo Lefkowitz is approved, and it is acceptable in quality and form for publication on microfilm and electronically: __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ __________________________________________________________________ Chair University of California, San Diego 2012 iii DEDICATION Ma and Ba, for your early indulgence and support. Matt and James, for choosing more practical callings. Roy, my love, for patiently sharing the ups and downs of this journey. iv EPIGRAPH It is foolish to tear one's hair in grief, as though sorrow would be made less by baldness. ~Cicero v TABLE OF CONTENTS Signature Page .............................................................................................................. iii Dedication .................................................................................................................... -

Lineage-Specific Evolution of the Vertebrate Otopetrin Gene Family Revealed by Comparative Genomic Analyses
Hurle et al. BMC Evolutionary Biology 2011, 11:23 http://www.biomedcentral.com/1471-2148/11/23 RESEARCHARTICLE Open Access Lineage-specific evolution of the vertebrate Otopetrin gene family revealed by comparative genomic analyses Belen Hurle1, Tomas Marques-Bonet2,3, Francesca Antonacci3, Inna Hughes4, Joseph F Ryan1, NISC Comparative Sequencing Program1,5, Evan E Eichler3, David M Ornitz6, Eric D Green1,5* Abstract Background: Mutations in the Otopetrin 1 gene (Otop1) in mice and fish produce an unusual bilateral vestibular pathology that involves the absence of otoconia without hearing impairment. The encoded protein, Otop1, is the only functionally characterized member of the Otopetrin Domain Protein (ODP) family; the extended sequence and structural preservation of ODP proteins in metazoans suggest a conserved functional role. Here, we use the tools of sequence- and cytogenetic-based comparative genomics to study the Otop1 and the Otop2-Otop3 genes and to establish their genomic context in 25 vertebrates. We extend our evolutionary study to include the gene mutated in Usher syndrome (USH) subtype 1G (Ush1g), both because of the head-to-tail clustering of Ush1g with Otop2 and because Otop1 and Ush1g mutations result in inner ear phenotypes. Results: We established that OTOP1 is the boundary gene of an inversion polymorphism on human chromosome 4p16 that originated in the common human-chimpanzee lineage more than 6 million years ago. Other lineage- specific evolutionary events included a three-fold expansion of the Otop genes in Xenopus tropicalis and of Ush1g in teleostei fish. The tight physical linkage between Otop2 and Ush1g is conserved in all vertebrates. -

Genome-Wide Analysis of Allele-Specific Expression Patterns in Seventeen Tissues of Korean Cattle (Hanwoo)
animals Article Genome-Wide Analysis of Allele-Specific Expression Patterns in Seventeen Tissues of Korean Cattle (Hanwoo) Kyu-Sang Lim 1 , Sun-Sik Chang 2, Bong-Hwan Choi 3, Seung-Hwan Lee 4, Kyung-Tai Lee 3 , Han-Ha Chai 3, Jong-Eun Park 3 , Woncheoul Park 3 and Dajeong Lim 3,* 1 Department of Animal Science, Iowa State University, Ames, IA 50011, USA; [email protected] 2 Hanwoo Research Institute, National Institute of Animal Science, Rural Development Administration, Pyeongchang 25340, Korea; [email protected] 3 Animal Genomics and Bioinformatics Division, National Institute of Animal Science, Rural Development Administration, Wanju 55365, Korea; [email protected] (B.-H.C.); [email protected] (K.-T.L.); [email protected] (H.-H.C.); [email protected] (J.-E.P.); [email protected] (W.P.) 4 Division of Animal and Dairy Science, Chungnam National University, Daejeon 34134, Korea; [email protected] * Correspondence: [email protected] Received: 26 July 2019; Accepted: 23 September 2019; Published: 26 September 2019 Simple Summary: Allele-specific expression (ASE) is the biased allelic expression of genetic variants within the gene. Recently, the next-generation sequencing (NGS) technologies allowed us to detect ASE genes at a transcriptome-wide level. It is essential for the understanding of animal development, cellular programming, and the effect on their complexity because ASE shows developmental, tissue, or species-specific patterns. However, these aspects of ASE still have not been annotated well in farm animals and most studies were conducted mainly at the fetal stages. Hence, the current study focuses on detecting ASE genes in 17 tissues in adult cattle. -

Propranolol-Mediated Attenuation of MMP-9 Excretion in Infants with Hemangiomas
Supplementary Online Content Thaivalappil S, Bauman N, Saieg A, Movius E, Brown KJ, Preciado D. Propranolol-mediated attenuation of MMP-9 excretion in infants with hemangiomas. JAMA Otolaryngol Head Neck Surg. doi:10.1001/jamaoto.2013.4773 eTable. List of All of the Proteins Identified by Proteomics This supplementary material has been provided by the authors to give readers additional information about their work. © 2013 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 eTable. List of All of the Proteins Identified by Proteomics Protein Name Prop 12 mo/4 Pred 12 mo/4 Δ Prop to Pred mo mo Myeloperoxidase OS=Homo sapiens GN=MPO 26.00 143.00 ‐117.00 Lactotransferrin OS=Homo sapiens GN=LTF 114.00 205.50 ‐91.50 Matrix metalloproteinase‐9 OS=Homo sapiens GN=MMP9 5.00 36.00 ‐31.00 Neutrophil elastase OS=Homo sapiens GN=ELANE 24.00 48.00 ‐24.00 Bleomycin hydrolase OS=Homo sapiens GN=BLMH 3.00 25.00 ‐22.00 CAP7_HUMAN Azurocidin OS=Homo sapiens GN=AZU1 PE=1 SV=3 4.00 26.00 ‐22.00 S10A8_HUMAN Protein S100‐A8 OS=Homo sapiens GN=S100A8 PE=1 14.67 30.50 ‐15.83 SV=1 IL1F9_HUMAN Interleukin‐1 family member 9 OS=Homo sapiens 1.00 15.00 ‐14.00 GN=IL1F9 PE=1 SV=1 MUC5B_HUMAN Mucin‐5B OS=Homo sapiens GN=MUC5B PE=1 SV=3 2.00 14.00 ‐12.00 MUC4_HUMAN Mucin‐4 OS=Homo sapiens GN=MUC4 PE=1 SV=3 1.00 12.00 ‐11.00 HRG_HUMAN Histidine‐rich glycoprotein OS=Homo sapiens GN=HRG 1.00 12.00 ‐11.00 PE=1 SV=1 TKT_HUMAN Transketolase OS=Homo sapiens GN=TKT PE=1 SV=3 17.00 28.00 ‐11.00 CATG_HUMAN Cathepsin G OS=Homo -

A Cell Line P53 Mutation Type UM
A Cell line p53 mutation Type UM-SCC 1 wt UM-SCC5 Exon 5, 157 GTC --> TTC Missense mutation by transversion (Valine --> Phenylalanine UM-SCC6 wt UM-SCC9 wt UM-SCC11A wt UM-SCC11B Exon 7, 242 TGC --> TCC Missense mutation by transversion (Cysteine --> Serine) UM-SCC22A Exon 6, 220 TAT --> TGT Missense mutation by transition (Tyrosine --> Cysteine) UM-SCC22B Exon 6, 220 TAT --> TGT Missense mutation by transition (Tyrosine --> Cysteine) UM-SCC38 Exon 5, 132 AAG --> AAT Missense mutation by transversion (Lysine --> Asparagine) UM-SCC46 Exon 8, 278 CCT --> CGT Missense mutation by transversion (Proline --> Alanine) B 1 Supplementary Methods Cell Lines and Cell Culture A panel of ten established HNSCC cell lines from the University of Michigan series (UM-SCC) was obtained from Dr. T. E. Carey at the University of Michigan, Ann Arbor, MI. The UM-SCC cell lines were derived from eight patients with SCC of the upper aerodigestive tract (supplemental Table 1). Patient age at tumor diagnosis ranged from 37 to 72 years. The cell lines selected were obtained from patients with stage I-IV tumors, distributed among oral, pharyngeal and laryngeal sites. All the patients had aggressive disease, with early recurrence and death within two years of therapy. Cell lines established from single isolates of a patient specimen are designated by a numeric designation, and where isolates from two time points or anatomical sites were obtained, the designation includes an alphabetical suffix (i.e., "A" or "B"). The cell lines were maintained in Eagle's minimal essential media supplemented with 10% fetal bovine serum and penicillin/streptomycin. -

Genome-Wide Approach to Identify Risk Factors for Therapy-Related Myeloid Leukemia
Leukemia (2006) 20, 239–246 & 2006 Nature Publishing Group All rights reserved 0887-6924/06 $30.00 www.nature.com/leu ORIGINAL ARTICLE Genome-wide approach to identify risk factors for therapy-related myeloid leukemia A Bogni1, C Cheng2, W Liu2, W Yang1, J Pfeffer1, S Mukatira3, D French1, JR Downing4, C-H Pui4,5,6 and MV Relling1,6 1Department of Pharmaceutical Sciences, The University of Tennessee, Memphis, TN, USA; 2Department of Biostatistics, The University of Tennessee, Memphis, TN, USA; 3Hartwell Center, The University of Tennessee, Memphis, TN, USA; 4Department of Pathology, The University of Tennessee, Memphis, TN, USA; 5Department of Hematology/Oncology St Jude Children’s Research Hospital, The University of Tennessee, Memphis, TN, USA; and 6Colleges of Medicine and Pharmacy, The University of Tennessee, Memphis, TN, USA Using a target gene approach, only a few host genetic risk therapy increases, the importance of identifying host factors for factors for treatment-related myeloid leukemia (t-ML) have been secondary neoplasms increases. defined. Gene expression microarrays allow for a more 4 genome-wide approach to assess possible genetic risk factors Because DNA microarrays interrogate multiple ( 10 000) for t-ML. We assessed gene expression profiles (n ¼ 12 625 genes in one experiment, they allow for a ‘genome-wide’ probe sets) in diagnostic acute lymphoblastic leukemic cells assessment of genes that may predispose to leukemogenesis. from 228 children treated on protocols that included leukemo- DNA microarray analysis of gene expression has been used to genic agents such as etoposide, 13 of whom developed t-ML. identify distinct expression profiles that are characteristic of Expression of 68 probes, corresponding to 63 genes, was different leukemia subtypes.13,14 Studies using this method have significantly related to risk of t-ML. -

Steroid-Dependent Regulation of the Oviduct: a Cross-Species Transcriptomal Analysis
University of Kentucky UKnowledge Theses and Dissertations--Animal and Food Sciences Animal and Food Sciences 2015 Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis Katheryn L. Cerny University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Cerny, Katheryn L., "Steroid-dependent regulation of the oviduct: A cross-species transcriptomal analysis" (2015). Theses and Dissertations--Animal and Food Sciences. 49. https://uknowledge.uky.edu/animalsci_etds/49 This Doctoral Dissertation is brought to you for free and open access by the Animal and Food Sciences at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Animal and Food Sciences by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

DNA Methylation Changes in Down Syndrome Derived Neural Ipscs Uncover Co-Dysregulation of ZNF and HOX3 Families of Transcription
Laan et al. Clinical Epigenetics (2020) 12:9 https://doi.org/10.1186/s13148-019-0803-1 RESEARCH Open Access DNA methylation changes in Down syndrome derived neural iPSCs uncover co- dysregulation of ZNF and HOX3 families of transcription factors Loora Laan1†, Joakim Klar1†, Maria Sobol1, Jan Hoeber1, Mansoureh Shahsavani2, Malin Kele2, Ambrin Fatima1, Muhammad Zakaria1, Göran Annerén1, Anna Falk2, Jens Schuster1 and Niklas Dahl1* Abstract Background: Down syndrome (DS) is characterized by neurodevelopmental abnormalities caused by partial or complete trisomy of human chromosome 21 (T21). Analysis of Down syndrome brain specimens has shown global epigenetic and transcriptional changes but their interplay during early neurogenesis remains largely unknown. We differentiated induced pluripotent stem cells (iPSCs) established from two DS patients with complete T21 and matched euploid donors into two distinct neural stages corresponding to early- and mid-gestational ages. Results: Using the Illumina Infinium 450K array, we assessed the DNA methylation pattern of known CpG regions and promoters across the genome in trisomic neural iPSC derivatives, and we identified a total of 500 stably and differentially methylated CpGs that were annotated to CpG islands of 151 genes. The genes were enriched within the DNA binding category, uncovering 37 factors of importance for transcriptional regulation and chromatin structure. In particular, we observed regional epigenetic changes of the transcription factor genes ZNF69, ZNF700 and ZNF763 as well as the HOXA3, HOXB3 and HOXD3 genes. A similar clustering of differential methylation was found in the CpG islands of the HIST1 genes suggesting effects on chromatin remodeling. Conclusions: The study shows that early established differential methylation in neural iPSC derivatives with T21 are associated with a set of genes relevant for DS brain development, providing a novel framework for further studies on epigenetic changes and transcriptional dysregulation during T21 neurogenesis. -

Cancer TNT Ashwin Ram 12/5/2017 Background: Chromatin Writers, Readers, Erasers
Cancer TNT Ashwin Ram 12/5/2017 Background: Chromatin Writers, Readers, Erasers writer effector eg. HAT, HMT reader eg. bromodomain eraser eg. HDAC, KDM The writer HAT1: A known H4 lysine 5,12 di-acetyltransferase writer siHAT1 siControl HAT1 H4 K12Ac H4 K5Ac actin Western blot for histone H4 modifications after control and HAT1 siRNA transfections. HAT1: EGF-stimulated immunoprecipitation specific specific - - HAT1 IgG HAT1 IgG - - R α non R α non EGF: + + + - - - WB: HAT1 Immunoprecipitation / WB to measure HAT1 levels +/- Heatmap of gene expression changes of all human histone EGF acetyltransferases +/- EGF and siRNA treatments shows HAT1 expression is EGF-dependent Working model of HAT1 : The oldest “new” histone acetyltransferase EGF EGFR plasma membrane HAT1 H4 H3 Rbap46/48 a nuclear membrane H4 H2A H3 H2B HAT1 S phase Surprise: HAT1 also binds (a few sites) on chromatin HAT1 ChipSeq signal sits on Hist1 locus on Chromosome 6 Read Depth HAT1 bound sites (zoom) HAT1 ChIP-seq peaks cluster at Read Depth histone H4 promoters. Hist1H2BE Hist1H4D Hist1H3D Hist1H4E Is HAT1 a transcription factor for its substrate (H4)? EGF EGFR plasma membrane HAT1 H4 H3 Rbap46/48 a nuclear membrane H4 H2A H3 H2B HAT1 S phase HAT1 is required for S-phase burst of histone H4 mRNA HAT1 Hist1H4B mRNA level 50 Rbap46 45 shCont-3 H4 40 shHAT1-A7 35 shHAT1-B6 30 25 B6 A7 - - 20 15 shHAT1 shHAT1 shControl 10 Gene actin)Expression (versus 5 HAT1 0 0 2 4 6 8 10 actin hours after release from double thymidine block G1 S G2/M HAT1 loss: Life with less histones EGF -

Differential Epigenomic and Transcriptomic Responses in Subcutaneous Adipose Tissue Between Low and High Responders to Caloric Restriction1–3
Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction1–3 Luigi Bouchard, Re´mi Rabasa-Lhoret, May Faraj, Marie-E`ve Lavoie, Jonathan Mill, Louis Pe´russe, and Marie-Claude Vohl ABSTRACT weight loss responses to caloric restriction show considerable Background: Caloric restriction is recommended for the treatment interindividual variability (7). Studies of genetically identical of obesity, but it is generally characterized by large interindividual monozygotic twins have been particularly useful in disentangling variability in responses. The factors affecting the magnitude of the role of environmental and heritable factors in determining the weight loss remain poorly understood. Epigenetic factors (ie, heri- degree of weight loss. It has been shown that within-pair changes table but reversible changes to genomic function that regulate gene in body fat variability after a caloric deficit is significantly lower expression independently of DNA sequence) may explain some of than between-pair variability, which suggests that genetic factors the interindividual variability seen in weight-loss responses. have an important influence on an individual’s response to caloric Objective: The objective was to determine whether epigenetics and deficit (8, 9). However, the concordance between twin pairs was gene expression changes may play a role in weight-loss responsiveness. not complete, which suggests that environmental factors or other Design: Overweight/obese postmenopausal women were recruited DNA sequence–independent mechanisms may be involved. for a standard 6-mo caloric restriction intervention. Abdominal sub- It has been suggested that monozygotic twin discordance for cutaneous adipose tissue biopsy samples were collected before (n = complex traits such as body weight could be accounted for by 14) and after (n = 14) intervention, and the epigenomic and tran- epigenetic factors (10, 11). -

Comprehensive Genome Methylation Analysis in Bladder Cancer: Identification and Validation of Novel Methylated Genes and Application of These As Urinary Tumor Markers
Published OnlineFirst July 25, 2011; DOI: 10.1158/1078-0432.CCR-10-2659 Clinical Cancer Human Cancer Biology Research Comprehensive Genome Methylation Analysis in Bladder Cancer: Identification and Validation of Novel Methylated Genes and Application of These as Urinary Tumor Markers Thomas Reinert1, Charlotte Modin1, Francisco M. Castano1, Philippe Lamy1, Tomasz K. Wojdacz4, Lise Lotte Hansen4, Carsten Wiuf3, Michael Borre2, Lars Dyrskjøt1, and Torben F. Ørntoft1 Abstract Purpose: Epigenetic alterations are common and can now be addressed in a parallel fashion. We investigated the methylation in bladder cancer with respect to location in genome, consistency, variation in metachronous tumors, impact on transcripts, chromosomal location, and usefulness as urinary markers. Experimental Design: A microarray assay was utilized to analyze methylation in 56 samples. Inde- pendent validation was conducted in 63 samples by a PCR-based method and bisulfite sequencing. The methylation levels in 174 urine specimens were quantified. Transcript levels were analyzed using expression microarrays and pathways were analyzed using dedicated software. Results: Global methylation patterns were established within and outside CpG islands. We validated methylation of the eight tumor markers genes ZNF154 (P < 0.0001), HOXA9 (P < 0.0001), POU4F2 (P < 0.0001), EOMES (P ¼ 0.0005), ACOT11 (P ¼ 0.0001), PCDHGA12 (P ¼ 0.0001), CA3 (P ¼ 0.0002), and PTGDR (P ¼ 0.0110), the candidate marker of disease progression TBX4 (P < 0.04), and other genes with stage-specific methylation. The methylation of metachronous tumors was stable and targeted to certain pathways. The correlation to expression was not stringent. Chromosome 21 showed most differential methylation (P < 0.0001) and specifically hypomethylation of keratins, which together with keratin-like proteins were epigenetically regulated.