Leuciscus Aspius) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Trends of Aquatic Alien Species Invasions in Ukraine
Aquatic Invasions (2007) Volume 2, Issue 3: 215-242 doi: http://dx.doi.org/10.3391/ai.2007.2.3.8 Open Access © 2007 The Author(s) Journal compilation © 2007 REABIC Research Article Trends of aquatic alien species invasions in Ukraine Boris Alexandrov1*, Alexandr Boltachev2, Taras Kharchenko3, Artiom Lyashenko3, Mikhail Son1, Piotr Tsarenko4 and Valeriy Zhukinsky3 1Odessa Branch, Institute of Biology of the Southern Seas, National Academy of Sciences of Ukraine (NASU); 37, Pushkinska St, 65125 Odessa, Ukraine 2Institute of Biology of the Southern Seas NASU; 2, Nakhimova avenue, 99011 Sevastopol, Ukraine 3Institute of Hydrobiology NASU; 12, Geroyiv Stalingrada avenue, 04210 Kiyv, Ukraine 4Institute of Botany NASU; 2, Tereschenkivska St, 01601 Kiyv, Ukraine E-mail: [email protected] (BA), [email protected] (AB), [email protected] (TK, AL), [email protected] (PT) *Corresponding author Received: 13 November 2006 / Accepted: 2 August 2007 Abstract This review is a first attempt to summarize data on the records and distribution of 240 alien species in fresh water, brackish water and marine water areas of Ukraine, from unicellular algae up to fish. A checklist of alien species with their taxonomy, synonymy and with a complete bibliography of their first records is presented. Analysis of the main trends of alien species introduction, present ecological status, origin and pathways is considered. Key words: alien species, ballast water, Black Sea, distribution, invasion, Sea of Azov introduction of plants and animals to new areas Introduction increased over the ages. From the beginning of the 19th century, due to The range of organisms of different taxonomic rising technical progress, the influence of man groups varies with time, which can be attributed on nature has increased in geometrical to general processes of phylogenesis, to changes progression, gradually becoming comparable in in the contours of land and sea, forest and dimensions to climate impact. -

Histopathological Effects and Toxicity of Atrazine Herbicide in Caspian Kutum, Rutilus Frisii Kutum, Fry
Iranian Journal of Fisheries Sciences 13(3) 702- 718 2014 Histopathological effects and toxicity of atrazine herbicide in Caspian Kutum, Rutilus frisii kutum, fry Khoshnood Z. 1*; Jamili Sh. 1; Khodabandeh S.2; Mashinchian Moradi A.1; Motallebi Moghanjoghi A.A.3 Received: June 2012 Accepted: May 2014 Abstract This study aimed to investigate the toxic effects of atrazine herbicide on the fry of Caspian Kutum (Rutilus frisii kutum, Kamensky, 1901). First the 96-h LC50 of the fry were exposed to atrazine at the concentration of 24.95 ppm was determined. Then the toxicity of this herbicide on Caspian kutum fry exposed to the concentration of 12.47ppm (1/2 LC50), for four days was measured and compared with a control group. Comparison of the length, weight and condition factor showed no significant differences between atrazine exposed and control group. The concentration of Na+, K+, Ca2+, Mg2+ and Cl- in the whole body of fry in control and atrazine exposure groups were as the following order: Ca2+>K+> Na +> Cl- >Mg2+ and Ca2+>Na+>K+>Mg2+>Cl-, respectively. Results showed that the concentration of all these ions were higher in atrazine exposure group than control group, except for Cl-, and the only significant differences was found in Na+ concentration. Major histopathological effects of atrazine on the gills were hyperplasia and thickening of the filaments, separation of the pavement cells of the lamellae epithelium from the pillar cells and swelling of the epithelial cells. Results of the present study showed that atrazine could affect the ion composition of the body, and caused major damages in gill epithelium even at sublethal concentration and acute exposure, but had no effects on the growth parameters. -

THE DEVELOPMENT of MESOZOIC SEDIMENTARY IBASINS AROUND the MARGINS of the NORTH ATLANTIC and THEIR HYDROCARBON POTENTIAL D.G. Masson and P.R
INTERNAL DOCUMENT IIL THE DEVELOPMENT OF MESOZOIC SEDIMENTARY IBASINS AROUND THE MARGINS OF THE NORTH ATLANTIC AND THEIR HYDROCARBON POTENTIAL D.G. Masson and P.R. Miles Internal Document No. 195 December 1983 [This document should not be cited in a published bibliography, and is supplied for the use of the recipient only]. iimsTit\jte of \ OCEANOGRAPHIC SCIENCES % INSTITUTE OF OCEANOGRAPHIC SCIENCES Wormley, Godalming, Surrey GU8 5UB (042-879-4141) (Director: Dr. A. S. Laughton, FRS) Bidston Observatory, Crossway, Birkenhead, Taunton, Mersey side L43 7RA Somerset TA1 2DW (051-653-8633) (0823-86211) (Assistant Director: Dr. D. E. Cartwright) (Assistant Director: M.J. Tucker) THE DEVELOPMENT OF MESOZOIC SEDIMENTARY BASINS AROUND THE MARGINS OF THE NORTH ATLANTIC AND THEIR HYDROCARBON POTENTIAL D.G. Masson and P.R. Miles Internal Document No. 195 December 1983 Work carried out under contract to the Department of Energy This document should not be cited in a published bibliography except as 'personal communication', and is supplied for the use of the recipient only. Institute of Oceanographic Sciences, Brook Road, Wormley, Surrey GU8 SUB ABSTRACT The distribution of Mesozoic basins around the margins of the North Atlantic has been summarised, and is discussed in terms of a new earliest Cretaceous palaeogeographic reconstruction for the North Atlantic area. The Late Triassic-Early Jurassic rift basins of Iberia, offshore eastern Canada and the continental shelf of western Europe are seen to be the fragments of a formerly coherent NE trending rift system which probably formed as a result of tensional stress between Europe, Africa and North America. The separation of Europe, North America and Iberia was preceded by a Late Jurassic-Early Cretaceous rifting phase which is clearly distinct from the earlier Mesozoic rifting episode and was little influenced by it. -

Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus Idus
REVIEWS IN FISHERIES SCIENCE & AQUACULTURE https://doi.org/10.1080/23308249.2020.1822280 REVIEW Review and Meta-Analysis of the Environmental Biology and Potential Invasiveness of a Poorly-Studied Cyprinid, the Ide Leuciscus idus Mehis Rohtlaa,b, Lorenzo Vilizzic, Vladimır Kovacd, David Almeidae, Bernice Brewsterf, J. Robert Brittong, Łukasz Głowackic, Michael J. Godardh,i, Ruth Kirkf, Sarah Nienhuisj, Karin H. Olssonh,k, Jan Simonsenl, Michał E. Skora m, Saulius Stakenas_ n, Ali Serhan Tarkanc,o, Nildeniz Topo, Hugo Verreyckenp, Grzegorz ZieRbac, and Gordon H. Coppc,h,q aEstonian Marine Institute, University of Tartu, Tartu, Estonia; bInstitute of Marine Research, Austevoll Research Station, Storebø, Norway; cDepartment of Ecology and Vertebrate Zoology, Faculty of Biology and Environmental Protection, University of Lodz, Łod z, Poland; dDepartment of Ecology, Faculty of Natural Sciences, Comenius University, Bratislava, Slovakia; eDepartment of Basic Medical Sciences, USP-CEU University, Madrid, Spain; fMolecular Parasitology Laboratory, School of Life Sciences, Pharmacy and Chemistry, Kingston University, Kingston-upon-Thames, Surrey, UK; gDepartment of Life and Environmental Sciences, Bournemouth University, Dorset, UK; hCentre for Environment, Fisheries & Aquaculture Science, Lowestoft, Suffolk, UK; iAECOM, Kitchener, Ontario, Canada; jOntario Ministry of Natural Resources and Forestry, Peterborough, Ontario, Canada; kDepartment of Zoology, Tel Aviv University and Inter-University Institute for Marine Sciences in Eilat, Tel Aviv, -

Aremu SO, Et Al. Putting the Spotlight on Opisthorchiasis: the Dread of the Western Siberian Copyright© Aremu SO, Et Al
Public Health Open Access MEDWIN PUBLISHERS ISSN: 2578-5001 Committed to Create Value for researchers Putting the Spotlight on Opisthorchiasis: The Dread of the Western Siberian Region Aremu SO1,3*, Zephaniah HS2, Onifade EO3, Fatoke B1 and Bademosi O4 Review Article 1Faculty of General Medicine, Siberian State Medical University, Tomsk, Russian Federation Volume 4 Issue 1 2Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria Received Date: February 17, 2020 3Department of Biological Science, Federal University of Agriculture, Makurdi Benue State, Published Date: March 10, 2020 Nigeria DOI: 10.23880/phoa-16000151 4Department of Public Health, University College Dublin, Ireland *Corresponding author: Stephen Olaide Aremu, Faculty of General Medicine, Siberian State Medical University, Tomsk, Russian Federation, Email: [email protected] Abstract Introduction: Opisthorchiasis is no doubt one of the most neglected infectious disease inspite of its huge medical importance in some parts of the World. The past decade have seen a resurgence of interests in research relating to this public health issue, however there is still a lot to be done. Social Model: Not many models have been explored in Western Siberia to deal with the opisthorchiasis epidemic when compared to the different models that have been used for other regions affected by similar disease. Life Cycle: The complex life cycle of Opisthorchis felineus prevalent among the aboriginal population of the Western Siberian because of their habit of eating raw or undercooked fresh has humans and other feline species as definitive host and is really Diagnosis and Treatment: Diagnosis involve the use of stool microscopy, other methods such as mAb ELISA, LAMP and so on water fish (Cyprinidae) which are intermediate host of the parasite. -

Effectiveness of FISK, an Invasiveness Screening Tool for Nonnative
Risk Analysis DOI: 10.1111/risa.12050 Effectiveness of FISK, an Invasiveness Screening Tool for Non-Native Freshwater Fishes, to Perform Risk Identification Assessments in the Iberian Peninsula David Almeida,1,2 Filipe Ribeiro,3,4 Pedro M. Leunda,5,6 Lorenzo Vilizzi,7 and Gordon H. Copp1,2,6,8,∗ Risk assessments are crucial for identifying and mitigating impacts from biological invasions. The Fish Invasiveness Scoring Kit (FISK) is a risk identification (screening) tool for fresh- water fishes consisting of two subject areas: biogeography/history and biology/ecology. Ac- cording to the outcomes, species can be classified under particular risk categories. The aim of this study was to apply FISK to the Iberian Peninsula, a Mediterranean climate region highly important for freshwater fish conservation due to a high level of endemism. In total, 89 fish species were assessed by three independent assessors. Results from receiver operating characteristic analysis showed that FISK can discriminate reliably between noninvasive and invasive fishes for Iberia, with a threshold of 20.25, similar to those obtained in several regions around the world. Based on mean scores, no species was categorized as “low risk,” 50 species as “medium risk,” 17 as “moderately high risk,” 11 as “high risk,” and 11 as “very high risk.” The highest scoring species was goldfish Carassius auratus. Mean certainty in response was above the category “mostly certain,” ranging from tinfoil barb Barbonymus schwanenfeldii with the lowest certainty to eastern mosquitofish Gambusia holbrooki with the highest level. Pair-wise comparison showed significant differences between one assessor and the other two on mean certainty, with these two assessors showing a high coincidence rate for the species categorization. -

NAME of SPECIES: Ide (Leuciscus Idus)
NAME OF SPECIES: Ide (Leuciscus idus) A. CURRENT STATUS AND DISTRIBUTION 1. In Wisconsin? a. YES NO b. Abundance: c. Geographic Range: d. Type of Waters Invaded (rivers, ponds, lakes, etc): (in other states) pools in rivers and slow-flowing or still water, ponds, lakes, estuaries e. Historical Status and Rate of Spread in Wisconsin: 2. Invasive in Similar Climate YES NO Zones Where: Maine, northeastern US, Pennsylvania (localized populations in these states) 3. Similar Habitat Invaded YES NO Elsewhere Where: 4. In Surrounding States YES NO Where: Reports of introductions from MN, IL, IN, OH, though it's unclear if any reproducing populations remain 5. Competitive Ability High: Tolerate a wide range of conditions Low: Reports of localized reproducing populations in a few locations, but unclear if any still exist. Poor records on this fish, so its true extent in the US is not clear. However, it's been here since the 1800s and has failed to thrive outside of a few small ponds, etc. B. ESTABLISHMENT POTENTIAL AND LIFE HISTORY TRAITS 1. Temperature: Range: 4 deg. C - 20 deg. C Upper lethal temps. for larvae and juveniles aclimated to 6 - 22 deg. C was 24 - 29 deg. C (was lower for fish aclimated to lower temps) 2. Spawning Temperature: Range: Begins at water temps from 5 - 14 deg. C, optimal temp. for egg development 12 - 18 deg. C 3. Number of Eggs: Range: large range reported throughout Europe, from 15,000 - 263,000 egg per female 4. Preferred Spawning weedy, shallow areas where adhesive eggs attach to stones or Substrate: vegetation 5. -

The Whitefin Gudgeon Romanogobio Belingi New for the Netherlands
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Lauterbornia Jahr/Year: 2005 Band/Volume: 2005_55 Autor(en)/Author(s): Soes Menno, Spaans Piet J., Veenvliet Paul Artikel/Article: The Whitefin gudgeon Romanogobio belingi new for the Netherlands. 141-144 ©Erik Mauch Verlag, Dinkelscherben, Deutschland,141 Download unter www.biologiezentrum.at Lauterbornia 55: 141-144, D-86424 Dinkelscherben, 2005-08-19 The Whitefin gudgeon Romanogobio belingi new for The Netherlands D. M. Soes, P. J. Spaans and P.Veenvliet With 2 figures Keywords: Romanogobio, Pisces, Waal, Rhine, The Netherlands, first record Schlagwörter: Romanogobio, Pisces, Waal, Rhein, Niederlande, Erstfund The whitefin gudgeon Romanogobia belingi (Lukasch, 1933) is recorded for he first time from The Netherlands 1 Introduction In the past years the fish fauna of the Dutch rivers belonging to the River Rhine system has been altered significantly. Species introduced to the Rhine system, such as Vimba (Vimba vimba), Asp (Aspius aspius) and Danube bream (Abramis sapa) are nowadays found in the Dutch waters (De Nie, 1996; Frey hof, et al., 2000; Van Emmerik, 2003). One Danubian species, the Tubenose goby (Proterorhinus marmoratus), invaded the River Rhine system via the Main-Danube-Canal and made it up to the Netherlands (Winter, 2002). Several other species of gobies are thought to follow (Freyhof, 2003). Already in 1998, Freyhof reported on another species in the River Rhine, which could be expected to occur in the Netherlands. This species, the White fin gudgeon (Romanogobio belingi (Lukasch, 1933), was found to be common and widespread in German parts of the River Rhine. -

The Black Sea Region — Shores and Delta
Black Sea region. page 1 European Environment Agency Europe’s biodiversity — biogeographical regions and seas Biogeographical regions in Europe The Black Sea Region — shores and delta Original contributions from ETC/NPB: Sophie Condé, Dominique Richard (coordinators) Nathalie Liamine (editor) Anne-Sophie Leclère (data collection and processing) Barbara Sotolargo (drafting) Ulla Pinborg (final co-editor) Map production: UNEP/GRID Warsaw (final production) Project manager: Tor-Björn Larsson, EEA ZooBoTech HB, Sweden, Linus Svensson (final edition) Black Sea region. page 2 Summary ............................................................................................................ 3 1. What are the main characteristics and trends of the Black Sea biogeographical region? ..................................................................................... 3 1.1 General characteristics.............................................................................. 3 1.1.1 Extent and limitations ............................................................................ 3 1.1.2 Geomorphological and topography ........................................................... 3 1.1.3 Soils .................................................................................................... 4 1.1.4 Climate ................................................................................................ 4 1.2 Present biodiversity status and trends: habitats, fauna and flora ............. 5 1.2.1 Habitats .............................................................................................. -

Effect of Weight on Osmoregulation Ability in Rutilus Frisii Kutum Fingerlings
African Journal of Biotechnology Vol. 11(12), pp. 3014-3021, 9 February, 2012 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.3144 ISSN 1684–5315 © 2012 Academic Journals Full Length Research Paper Effect of weight on osmoregulation ability in Rutilus frisii kutum fingerlings Seyed Ali Hosseini1, Che Roos Saad1*, Seyed Ahmad Hosseini2, Mohammad Sayyad Bourani3, Hassan Mohd Daud4 and Sharr Azni Harmin5 1Department of Aquaculture, Faculty of Agriculture, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia. 2Kermanshah Branch, Islamic Azad University, Kermanshah, Iran. 3National Inland Waters Aquaculture Institute, Aquaculture Department, P.O. Box 66, Bandar Anzali, Iran. 4Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia. 5Center for Land and Aquatic Technology Faculty of Science and Biotechnology, University Selangor, 40000 Shah Alam, Selangor, Malaysia. Accepted 28 December, 2011 Experiments were conducted to study the downstream migratory behavior and effects of weight on osmoregulation ability of hatchery-reared Rutilus frisii kutum fingerlings during adaptation to the seawater. Accordingly, blood osmotic pressure regulation ability in kutum fingerlings with weights of 1, 3, 5 and 7 g in three different salinities, 13 ppt (Caspian Sea salinity), 7 ppt (estuarine area) and 0.3 ppt (freshwater) were assessed. The blood samples were collected before being transferred as control (freshwater) and after exposure to the sea and estuarine water in a period of up to 336 h by a pooling method. The results indicate that only 3, 5 and 7 g kutum are able to adapt to salinity of 7 and 13 ppt since they maintained the osmolarity. -

Resolving Cypriniformes Relationships Using an Anchored Enrichment Approach Carla C
Stout et al. BMC Evolutionary Biology (2016) 16:244 DOI 10.1186/s12862-016-0819-5 RESEARCH ARTICLE Open Access Resolving Cypriniformes relationships using an anchored enrichment approach Carla C. Stout1*†, Milton Tan1†, Alan R. Lemmon2, Emily Moriarty Lemmon3 and Jonathan W. Armbruster1 Abstract Background: Cypriniformes (minnows, carps, loaches, and suckers) is the largest group of freshwater fishes in the world (~4300 described species). Despite much attention, previous attempts to elucidate relationships using molecular and morphological characters have been incongruent. In this study we present the first phylogenomic analysis using anchored hybrid enrichment for 172 taxa to represent the order (plus three out-group taxa), which is the largest dataset for the order to date (219 loci, 315,288 bp, average locus length of 1011 bp). Results: Concatenation analysis establishes a robust tree with 97 % of nodes at 100 % bootstrap support. Species tree analysis was highly congruent with the concatenation analysis with only two major differences: monophyly of Cobitoidei and placement of Danionidae. Conclusions: Most major clades obtained in prior molecular studies were validated as monophyletic, and we provide robust resolution for the relationships among these clades for the first time. These relationships can be used as a framework for addressing a variety of evolutionary questions (e.g. phylogeography, polyploidization, diversification, trait evolution, comparative genomics) for which Cypriniformes is ideally suited. Keywords: Fish, High-throughput -
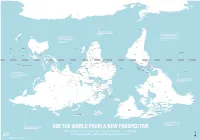
Is Map Is Just As Accurate As the One We're All Used To
ROSS SEA WEDDELL SEA AMUNDSEN SEA ANTARCTICA BELLINGSHAUSEN SEA AMERY ICE SHELF SOUTHERN OCEAN SOUTHERN OCEAN SOUTHERN OCEAN SCOTIA SEA DRAKE PASSAGE FALKLAND ISLANDS Stanley (U.K.) THE RATIO OF LAND TO WATER IN THE SOUTHERN HEMISPHERE BY THE TIME EUROPEANS ADOPTED IS 1 TO 5 THE NORTH-POINTING COMPASS, PTOLEMY WAS A HELLENIC Wellington THEY WERE ALREADY EXPERIENCED NEW TASMAN SEA ZEALAND CARTOGRAPHER WHOSE WORK CHILE IN NAVIGATING WITH REFERENCE TO IN THE SECOND CENTURY A.D. ARGENTINA THE NORTH STAR Canberra Buenos Santiago GREAT AUSTRALIAN BIGHT POPULARIZED NORTH-UP Montevideo Aires SOUTH PACIFIC OCEAN SOUTH ATLANTIC OCEAN URUGUAY ORIENTATION SOUTH AFRICA Maseru LESOTHO SWAZILAND Mbabane Maputo Asunción AUSTRALIA Pretoria Gaborone NEW Windhoek PARAGUAY TONGA Nouméa CALEDONIA BOTSWANA Saint Denis NAMIBIA Nuku’Alofa (FRANCE) MAURITIUS Port Louis Antananarivo MOZAMBIQUE CHANNEL ZIMBABWE Suva Port Vila MADAGASCAR VANUATU MOZAMBIQUE Harare La Paz INDIAN OCEAN Brasília LAKE SOUTH PACIFIC OCEAN FIJI Lusaka TITICACA CORAL SEA GREAT GULF OF BARRIER CARPENTARIA Lilongwe ZAMBIA BOLIVIA FRENCH POLYNESIA Apia REEF LAKE (FRANCE) SAMOA TIMOR SEA COMOROS NYASA ANGOLA Lima Moroni MALAWI Honiara ARAFURA SEA BRAZIL TIMOR LESTE PERU Funafuti SOLOMON Port Dili Luanda ISLANDS Moresby LAKE Dodoma TANGANYIKA TUVALU PAPUA Jakarta SEYCHELLES TANZANIA NEW GUINEA Kinshasa Victoria BURUNDI Bujumbura DEMOCRATIC KIRIBATI Brazzaville Kigali REPUBLIC LAKE OF THE CONGO SÃO TOMÉ Nairobi RWANDA GABON ECUADOR EQUATOR INDONESIA VICTORIA REP. OF AND PRINCIPE KIRIBATI EQUATOR