Complexity, Sphericity, and Ordering of Regular and Semiregular Polyhedra
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A New Calculable Orbital-Model of the Atomic Nucleus Based on a Platonic-Solid Framework
A new calculable Orbital-Model of the Atomic Nucleus based on a Platonic-Solid framework and based on constant Phi by Dipl. Ing. (FH) Harry Harry K. K.Hahn Hahn / Germany ------------------------------ 12. December 2020 Abstract : Crystallography indicates that the structure of the atomic nucleus must follow a crystal -like order. Quasicrystals and Atomic Clusters with a precise Icosahedral - and Dodecahedral structure indi cate that the five Platonic Solids are the theoretical framework behind the design of the atomic nucleus. With my study I advance the hypothesis that the reference for the shell -structure of the atomic nucleus are the Platonic Solids. In my new model of the atomic nucleus I consider the central space diagonals of the Platonic Solids as the long axes of Proton - or Neutron Orbitals, which are similar to electron orbitals. Ten such Proton- or Neutron Orbitals form a complete dodecahedral orbital-stru cture (shell), which is the shell -type with the maximum number of protons or neutrons. An atomic nucleus therefore mainly consists of dodecahedral shaped shells. But stable Icosahedral- and Hexagonal-(cubic) shells also appear in certain elements. Consta nt PhI which directly appears in the geometry of the Dodecahedron and Icosahedron seems to be the fundamental constant that defines the structure of the atomic nucleus and the structure of the wave systems (orbitals) which form the atomic nucelus. Albert Einstein wrote in a letter that the true constants of an Universal Theory must be mathematical constants like Pi (π) or e. My mathematical discovery described in chapter 5 shows that all irrational square roots of the natural numbers and even constant Pi (π) can be expressed with algebraic terms that only contain constant Phi (ϕ) and 1 Therefore it is logical to assume that constant Phi, which also defines the structure of the Platonic Solids must be the fundamental constant that defines the structure of the atomic nucleus. -

Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc
Metal-Metal (MM) Bond Distances and Bond Orders in Binuclear Metal Complexes of the First Row Transition Metals Titanium Through Zinc Richard H. Duncan Lyngdoh*,a, Henry F. Schaefer III*,b and R. Bruce King*,b a Department of Chemistry, North-Eastern Hill University, Shillong 793022, India B Centre for Computational Quantum Chemistry, University of Georgia, Athens GA 30602 ABSTRACT: This survey of metal-metal (MM) bond distances in binuclear complexes of the first row 3d-block elements reviews experimental and computational research on a wide range of such systems. The metals surveyed are titanium, vanadium, chromium, manganese, iron, cobalt, nickel, copper, and zinc, representing the only comprehensive presentation of such results to date. Factors impacting MM bond lengths that are discussed here include (a) n+ the formal MM bond order, (b) size of the metal ion present in the bimetallic core (M2) , (c) the metal oxidation state, (d) effects of ligand basicity, coordination mode and number, and (e) steric effects of bulky ligands. Correlations between experimental and computational findings are examined wherever possible, often yielding good agreement for MM bond lengths. The formal bond order provides a key basis for assessing experimental and computationally derived MM bond lengths. The effects of change in the metal upon MM bond length ranges in binuclear complexes suggest trends for single, double, triple, and quadruple MM bonds which are related to the available information on metal atomic radii. It emerges that while specific factors for a limited range of complexes are found to have their expected impact in many cases, the assessment of the net effect of these factors is challenging. -

How Platonic and Archimedean Solids Define Natural Equilibria of Forces for Tensegrity
How Platonic and Archimedean Solids Define Natural Equilibria of Forces for Tensegrity Martin Friedrich Eichenauer The Platonic and Archimedean solids are a well-known vehicle to describe Research Assistant certain phenomena of our surrounding world. It can be stated that they Technical University Dresden define natural equilibria of forces, which can be clarified particularly Faculty of Mathematics Institute of Geometry through the packing of spheres. [1][2] To solve the problem of the densest Germany packing, both geometrical and mechanical approach can be exploited. The mechanical approach works on the principle of minimal potential energy Daniel Lordick whereas the geometrical approach searches for the minimal distances of Professor centers of mass. The vertices of the solids are given by the centers of the Technical University Dresden spheres. Faculty of Geometry Institute of Geometry If we expand this idea by a contrary force, which pushes outwards, we Germany obtain the principle of tensegrity. We can show that we can build up regular and half-regular polyhedra by the interaction of physical forces. Every platonic and Archimedean solid can be converted into a tensegrity structure. Following this, a vast variety of shapes defined by multiple solids can also be obtained. Keywords: Platonic Solids, Archimedean Solids, Tensegrity, Force Density Method, Packing of Spheres, Modularization 1. PLATONIC AND ARCHIMEDEAN SOLIDS called “kissing number” problem. The kissing number problem is asking for the maximum possible number of Platonic and Archimedean solids have systematically congruent spheres, which touch another sphere of the been described in the antiquity. They denominate all same size without overlapping. In three dimensions the convex polyhedra with regular faces and uniform vertices kissing number is 12. -
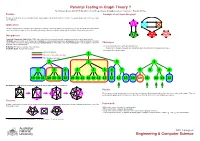
Parental Testing in Graph Theory?
Parental Testing in Graph Theory ? By S Narjess Afzaly,∗ ANU CSIT Algorithm & Data Group, [email protected]. Supervisor: Brendan McKay.∗ Problem A sample of an irreducible graph To make a complete list of non-isomorphic simple quartic graphs with the given number of vertices. In a quartic graph, each vertex has exactly four neighbours. Applications Finding counterexamples, verifying some conjectures or refining some proofs whether in graph theory or in any other field where the problem can be modelled as a graph such as: chemistry, web mining, national security, the railway map, the electrical circuit and neural science. Our approach Canonical Construction Path (McKay 1998): A general method to recursively generate combinatorial objects in which larger objects, (Children), are constructed out of smaller ones, (Parents), by some well-defined operation, (extension), and avoiding constructing isomorphic copies at each construction step by defining a unique genuine parent for each graph. Hence a generated graph is only accepted if it has been Challenges extended from its genuine parent. • To avoid isomorphic copies when generating the list: Reduction: The inverse operation of the extension. Irreducible graph: A graph with no parent. The definition of the genuine parent can substantially affect the efficiency of the generation process. • Generating all irreducible graphs. Genuine parent 1 Has an isomorphic sibling Not genuine parent 2 2 3 4 5 5 6 5 5 6 6 7 5 8 9 6 10 10 11 10 10 11 12 10 10 11 10 10 11 12 12 12 13 10 10 11 10 13 12 Our Reduction: Deleting a vertex and adding two extra disjoint edges between its neighbours. -

Fast Generation of Planar Graphs (Expanded Version)
Fast generation of planar graphs (expanded version) Gunnar Brinkmann∗ Brendan D. McKay† Department of Applied Mathematics Department of Computer Science and Computer Science Australian National University Ghent University Canberra ACT 0200, Australia B-9000 Ghent, Belgium [email protected] [email protected] Abstract The program plantri is the fastest isomorph-free generator of many classes of planar graphs, including triangulations, quadrangulations, and convex polytopes. Many applications in the natural sciences as well as in mathematics have appeared. This paper describes plantri’s principles of operation, the basis for its efficiency, and the recursive algorithms behind many of its capabilities. In addition, we give many counts of isomorphism classes of planar graphs compiled using plantri. These include triangulations, quadrangulations, convex polytopes, several classes of cubic and quartic graphs, and triangulations of disks. This paper is an expanded version of the paper “Fast generation of planar graphs” to appeared in MATCH. The difference is that this version has substantially more tables. 1 Introduction The program plantri can rapidly generate many classes of graphs embedded in the plane. Since it was first made available, plantri has been used as a tool for research of many types. We give only a partial list, beginning with masters theses [37, 40] and PhD theses [32, 42]. Applications have appeared in chemistry [8, 21, 28, 39, 44], in crystallography [19], in physics [25], and in various areas of mathematics [1, 2, 7, 20, 22, 24, 41]. ∗Research supported in part by the Australian National University. †Research supported by the Australian Research Council. 1 The purpose of this paper is to describe the facilities of plantri and to explain in general terms the method of operation. -

Structure and Bonding of New Boron and Carbon Superpolyhedra
Structural Chemistry (2019) 30:805–814 https://doi.org/10.1007/s11224-019-1279-5 ORIGINAL RESEARCH Structure and bonding of new boron and carbon superpolyhedra Olga A. Gapurenko1 & Ruslan M. Minyaev1 & Nikita S. Fedik2 & Vitaliy V. Koval1 & Alexander I. Boldyrev2 & Vladimir I. Minkin1 Received: 16 November 2018 /Accepted: 1 January 2019 /Published online: 10 January 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Using the DFT methods, we computationally predict the stability of cage compounds E4nRn (E = B, C; R = H, F; n = 4, 8, 12, 24) based on Platonic bodies and Archimedean polyhedrons in which all vertices are replaced by tetrahedral E4R fragments. Cage compounds B60R12 and C60 with pyramidal units B5RorC5 are also examined and it is shown that only boron compounds are stable. The nature of chemical bonding in the discussed compounds is analyzed using the AdNDP and NBO methods. The hydrocarbons have classical 2c-2e C-C σ-bonds, while the boron compounds are formed by the polyhedral units with the delocalized multicenter bonds which connected three and more boron atoms. The new example of spherical aromaticity accord- 2 ing to the 2(N+1) rule in the case of B16F4 with multicenter 16c-2e bonds are revealed. Stable compound B60H12 contains 12 5c- 2e B-B bonds. Keywords Сage clusters . Chemical bonding . 3c-2e bond . Spherical aromaticity . AdNDP . NBO Construction of novel allotropic forms of carbon based on was proposed [1] as the system with the same symmetry as the tetrahedrane- and cubane-like building blocks was pro- sp3-carbon to replace the carbon atoms in the diamond posed by Burdett and Lee [1] and by Johnston and lattice. -

Smallest Cubic and Quartic Graphs with a Given Number of Cutpoints and Bridges Gary Chartrand
Old Dominion University ODU Digital Commons Mathematics & Statistics Faculty Publications Mathematics & Statistics 1982 Smallest Cubic and Quartic Graphs With a Given Number of Cutpoints and Bridges Gary Chartrand Farrokh Saba John K. Cooper Jr. Frank Harary Curtiss E. Wall Old Dominion University Follow this and additional works at: https://digitalcommons.odu.edu/mathstat_fac_pubs Part of the Mathematics Commons Repository Citation Chartrand, Gary; Saba, Farrokh; Cooper, John K. Jr.; Harary, Frank; and Wall, Curtiss E., "Smallest Cubic and Quartic Graphs With a Given Number of Cutpoints and Bridges" (1982). Mathematics & Statistics Faculty Publications. 76. https://digitalcommons.odu.edu/mathstat_fac_pubs/76 Original Publication Citation Chartrand, G., Saba, F., Cooper, J. K., Harary, F., & Wall, C. E. (1982). Smallest cubic and quartic graphs with a given number of cutpoints and bridges. International Journal of Mathematics and Mathematical Sciences, 5(1), 41-48. doi:10.1155/s0161171282000052 This Article is brought to you for free and open access by the Mathematics & Statistics at ODU Digital Commons. It has been accepted for inclusion in Mathematics & Statistics Faculty Publications by an authorized administrator of ODU Digital Commons. For more information, please contact [email protected]. I nternat. J. Math. & Math. Sci. Vol. 5 No. (1982)41-48 41 SMALLEST CUBIC AND QUARTIC GRAPHS WITH A GIVEN NUMBER OF CUTPOINTS AND BRIDGES GARY CHARTRAND and FARROKH SABA Department of Mathematics, Western Michigan University Kalamazoo, Michigan 49008 U. S .A. JOHN K. COOPER, JR. Department of Mathematics, Eastern Michigan University Ypsilanti, Michigan 48197 U.S.A. FRANK HARARY Department of Mathematics, University of Michigan Ann Arbor, Michigan 48104 U.S.A. -

Distance-Two Colorings of Barnette Graphs
CCCG 2018, Winnipeg, Canada, August 8{10, 2018 Distance-Two Colorings of Barnette Graphs Tom´asFeder∗ Pavol Helly Carlos Subiz Abstract that the conjectures hold on the intersection of the two classes, i.e., that tri-connected cubic planar graphs with Barnette identified two interesting classes of cubic poly- all face sizes 4 or 6 are Hamiltonian. When all faces hedral graphs for which he conjectured the existence of have sizes 5 or 6, this was a longstanding open prob- a Hamiltonian cycle. Goodey proved the conjecture for lem, especially since these graphs (tri-connected cubic the intersection of the two classes. We examine these planar graphs with all face sizes 5 or 6) are the popu- classes from the point of view of distance-two color- lar fullerene graphs [8]. The second conjecture has now ings. A distance-two r-coloring of a graph G is an as- been affirmatively resolved in full [18]. For the first con- signment of r colors to the vertices of G so that any jecture, two of the present authors have shown in [10] two vertices at distance at most two have different col- that if the conjecture is false, then the Hamiltonicity ors. Note that a cubic graph needs at least four colors. problem for tri-connected cubic planar graphs is NP- The distance-two four-coloring problem for cubic planar complete. In view of these results and conjectures, in graphs is known to be NP-complete. We claim the prob- this paper we call bipartite tri-connected cubic planar lem remains NP-complete for tri-connected bipartite graphs type-one Barnette graphs; we call cubic plane cubic planar graphs, which we call type-one Barnette graphs with all face sizes 3; 4; 5 or 6 type-two Barnette graphs, since they are the first class identified by Bar- graphs; and finally we call cubic plane graphs with all nette. -

On Extremums of Sums of Powered Distances to a Finite Set of Points
ON EXTREMUMS OF SUMS OF POWERED DISTANCES TO A FINITE SET OF POINTS NIKOLAI NIKOLOV AND RAFAEL RAFAILOV Abstract. In this paper we investigate the extremal properties of the sum n X λ jMAij ; i=1 where Ai are vertices of a regular simplex, a cross-polytope (orthoplex) or a cube and M varies on a sphere concentric to the sphere circumscribed around one of the given polytopes. We give full characterization for which points on Γ the extremal values of the sum are obtained in 3 terms of λ. In the case of the regular dodecahedron and icosahedron in R we obtain results for which values of λ the corresponding sum is independent of the position of M on Γ. We use elementary analytic and purely geometric methods. 1. Introduction We investigate problems of the extremality of sums of the kind n X λ (1.1) jMAij ; i=1 where Ai are vertices of a regular simplex, a cross-polytope (orthoplex) or a cube and M varies on a sphere concentric to the sphere circumscribed around one of the given polytopes. Such questions arise frequently as physical problems. The case λ = −1 is equivalent to finding the points on the sphere with maximal and minimal potential. There is an extensive list of articles on the topic of finding extremal point configurations on the sphere with respect to some potential function [2], [3], and an experimental approach was presented in [4]. Later the question arised to find extremums on the sphere with respect to some potential function and fixed base points. -

Ring-Opening Alkyne Metathesis Methods for Functional Conjugated Polymer Synthesis by Stephen W. Von Kugelgen a Dissertation
Ring-Opening Alkyne Metathesis Methods For Functional Conjugated Polymer Synthesis by Stephen W. von Kugelgen A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Chemistry in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Felix R. Fischer, Chair Professor Robert G. Bergman Professor Phillip B. Messersmith Fall 2018 Ring-Opening Alkyne Metathesis Methods For Functional Conjugated Polymer Synthesis Copyright 2018 by Stephen W. von Kugelgen 1 Abstract Ring-Opening Alkyne Metathesis Methods For Functional Conjugated Polymer Synthesis by Stephen W. von Kugelgen Doctor of Philosophy in Chemistry University of California, Berkeley Professor Felix R. Fischer, Chair Since its discovery in the mid 20th century, most applications of alkyne metathesis have relied on thermodynamics to control product distributions. Ring-opening alkyne metathe- sis polymerization (ROAMP), in contrast, requires the kinetic product of metathesis of a strained, cyclic alkyne monomer to give a living, chain-growth polymerization (Chapter 1, Introduction). This living polymerization of conjugated alkyne-containing monomers has the potential to access a wide range of functional poly(arylene ethynylene) materials with excep- tional control over length, dispersity, topology, and endgroups. To this end, we demonstrate the first ROAMP synthesis of conjugated poly(ortho-phenylene ethynylene) and elucidate a mechanistic description of the reaction to understand the enabling catalyst selectivity and unexpectely find that initiator sterics dictate endgroup fidelity and polymer topology (Chap- ter 2). To disentangle the role of steric and electronic factors in initiator performance, we describe a novel synthetic method that gives a series of isosteric benzylidyne catalysts which exhibit a strong, deterministic electronic effect on both ROAMP initiation rates and polymer endgroup fidelity (Chapter 3). -

7 Dee's Decad of Shapes and Plato's Number.Pdf
Dee’s Decad of Shapes and Plato’s Number i © 2010 by Jim Egan. All Rights reserved. ISBN_10: ISBN-13: LCCN: Published by Cosmopolite Press 153 Mill Street Newport, Rhode Island 02840 Visit johndeetower.com for more information. Printed in the United States of America ii Dee’s Decad of Shapes and Plato’s Number by Jim Egan Cosmopolite Press Newport, Rhode Island C S O S S E M R O P POLITE “Citizen of the World” (Cosmopolite, is a word coined by John Dee, from the Greek words cosmos meaning “world” and politês meaning ”citizen”) iii Dedication To Plato for his pursuit of “Truth, Goodness, and Beauty” and for writing a mathematical riddle for Dee and me to figure out. iv Table of Contents page 1 “Intertransformability” of the 5 Platonic Solids 15 The hidden geometric solids on the Title page of the Monas Hieroglyphica 65 Renewed enthusiasm for the Platonic and Archimedean solids in the Renaissance 87 Brief Biography of Plato 91 Plato’s Number(s) in Republic 8:546 101 An even closer look at Plato’s Number(s) in Republic 8:546 129 Plato shows his love of 360, 2520, and 12-ness in the Ideal City of “The Laws” 153 Dee plants more clues about Plato’s Number v vi “Intertransformability” of the 5 Platonic Solids Of all the polyhedra, only 5 have the stuff required to be considered “regular polyhedra” or Platonic solids: Rule 1. The faces must be all the same shape and be “regular” polygons (all the polygon’s angles must be identical). -

Alkyne-Cobalt-Clusters
COBALT -CLUSTER MEDIATED PROPARGYL CATION AND RADICAI.J REARRANGEMENTS ALKYNE-COBALT-CLUSTERS: SYNTHESES, STRUCTURES AND REARRANGEMENTS OF METAL··STABILIZED PROPARGYL CATIONS AND RADICALS By JOHN H. KALDIS, B.Sc. A Thesis Submitted to the School of Graduate Studies In Partial Fulfillment ofthe Requirements for the Degree Doctor of Philosophy McMaster University © Copyright by John H. Kaldis, August 2003 DOCTOR OF PHILOSOPHY (2003) McMaster University (Chemistry) Hamilton, Ontario TITLE: Alkyne-Cobalt-Clusters: Syntheses, Structures and Rearrangements of Metal-Stabilized Propargyl Cations and Radicals AUTHOR: John H. Kaldis, B.Sc. (University of Western Ontario) SUPERVISOR: Dr. Michael J. McGlinchey NUMBER OF PAGES: XV, 192 11 Abstract Cobalt-clusters are versatile reagents in organometallic chemistry. Their ability to protect an alkyne allows one to selectively manipulate a ligand without undergoing a competitive reaction from the alkyne. Cobalt-clusters geometrically modify linear alkynes to 136-145° degrees, thereby allowing for some non-traditional alkynyl chemistry to occur. In particular, the focus of this dissertation lies upon the chemistry of cobalt-complexed propargyl alkynols, the ability of cobalt to stabilize neighbouring cations generated from these alcohols, and the chemistry that can be accomplished by altering the steric and electronic effects. We have chosen to study the possibility of inducing migration of various substituents from one terminus of the cobalt-complexed alkyne to the alcoholic site ofthe propargyl group via protonation ofthe desired complex. While examining various silanes, and altering the propargyl alcohol itself, we have considered both steric and electronic effects, thereby determining the idealized conditions for such transfers to occur. Furthermore, in our attempts to successfully apply these migrations to several systems, we have acquired a diverse synthetic knowledge of propargyl cobalt-clusters and their intricate reactivity.