Ecological Guild Evolution and the Discovery of the World’S Smallest Vertebrate
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Catalogue of the Amphibians of Venezuela: Illustrated and Annotated Species List, Distribution, and Conservation 1,2César L
Mannophryne vulcano, Male carrying tadpoles. El Ávila (Parque Nacional Guairarepano), Distrito Federal. Photo: Jose Vieira. We want to dedicate this work to some outstanding individuals who encouraged us, directly or indirectly, and are no longer with us. They were colleagues and close friends, and their friendship will remain for years to come. César Molina Rodríguez (1960–2015) Erik Arrieta Márquez (1978–2008) Jose Ayarzagüena Sanz (1952–2011) Saúl Gutiérrez Eljuri (1960–2012) Juan Rivero (1923–2014) Luis Scott (1948–2011) Marco Natera Mumaw (1972–2010) Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 13(1) [Special Section]: 1–198 (e180). Catalogue of the amphibians of Venezuela: Illustrated and annotated species list, distribution, and conservation 1,2César L. Barrio-Amorós, 3,4Fernando J. M. Rojas-Runjaic, and 5J. Celsa Señaris 1Fundación AndígenA, Apartado Postal 210, Mérida, VENEZUELA 2Current address: Doc Frog Expeditions, Uvita de Osa, COSTA RICA 3Fundación La Salle de Ciencias Naturales, Museo de Historia Natural La Salle, Apartado Postal 1930, Caracas 1010-A, VENEZUELA 4Current address: Pontifícia Universidade Católica do Río Grande do Sul (PUCRS), Laboratório de Sistemática de Vertebrados, Av. Ipiranga 6681, Porto Alegre, RS 90619–900, BRAZIL 5Instituto Venezolano de Investigaciones Científicas, Altos de Pipe, apartado 20632, Caracas 1020, VENEZUELA Abstract.—Presented is an annotated checklist of the amphibians of Venezuela, current as of December 2018. The last comprehensive list (Barrio-Amorós 2009c) included a total of 333 species, while the current catalogue lists 387 species (370 anurans, 10 caecilians, and seven salamanders), including 28 species not yet described or properly identified. Fifty species and four genera are added to the previous list, 25 species are deleted, and 47 experienced nomenclatural changes. -

Fauna of Australia 2A
FAUNA of AUSTRALIA 9. FAMILY MICROHYLIDAE Thomas C. Burton 1 9. FAMILY MICROHYLIDAE Pl 1.3. Cophixalus ornatus (Microhylidae): usually found in leaf litter, this tiny frog is endemic to the wet tropics of northern Queensland. [H. Cogger] 2 9. FAMILY MICROHYLIDAE DEFINITION AND GENERAL DESCRIPTION The Microhylidae is a family of firmisternal frogs, which have broad sacral diapophyses, one or more transverse folds on the surface of the roof of the mouth, and a unique slip to the abdominal musculature, the m. rectus abdominis pars anteroflecta (Burton 1980). All but one of the Australian microhylids are small (snout to vent length less than 35 mm), and all have procoelous vertebrae, are toothless and smooth-bodied, with transverse grooves on the tips of their variously expanded digits. The terminal phalanges of fingers and toes of all Australian microhylids are T-shaped or Y-shaped (Pl. 1.3) with transverse grooves. The Microhylidae consists of eight subfamilies, of which two, the Asterophryinae and Genyophryninae, occur in the Australopapuan region. Only the Genyophryninae occurs in Australia, represented by Cophixalus (11 species) and Sphenophryne (five species). Two newly discovered species of Cophixalus await description (Tyler 1989a). As both genera are also represented in New Guinea, information available from New Guinean species is included in this chapter to remedy deficiencies in knowledge of the Australian fauna. HISTORY OF DISCOVERY The Australian microhylids generally are small, cryptic and tropical, and so it was not until 100 years after European settlement that the first species, Cophixalus ornatus, was collected, in 1888 (Fry 1912). As the microhylids are much more prominent and diverse in New Guinea than in Australia, Australian specimens have been referred to New Guinean species from the time of the early descriptions by Fry (1915), whilst revisions by Parker (1934) and Loveridge (1935) minimised the extent of endemism in Australia. -

The Impact of Anchored Phylogenomics and Taxon Sampling on Phylogenetic Inference in Narrow-Mouthed Frogs (Anura, Microhylidae)
Cladistics Cladistics (2015) 1–28 10.1111/cla.12118 The impact of anchored phylogenomics and taxon sampling on phylogenetic inference in narrow-mouthed frogs (Anura, Microhylidae) Pedro L.V. Pelosoa,b,*, Darrel R. Frosta, Stephen J. Richardsc, Miguel T. Rodriguesd, Stephen Donnellane, Masafumi Matsuif, Cristopher J. Raxworthya, S.D. Bijug, Emily Moriarty Lemmonh, Alan R. Lemmoni and Ward C. Wheelerj aDivision of Vertebrate Zoology (Herpetology), American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024, USA; bRichard Gilder Graduate School, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024, USA; cHerpetology Department, South Australian Museum, North Terrace, Adelaide, SA 5000, Australia; dDepartamento de Zoologia, Instituto de Biociencias,^ Universidade de Sao~ Paulo, Rua do Matao,~ Trav. 14, n 321, Cidade Universitaria, Caixa Postal 11461, CEP 05422-970, Sao~ Paulo, Sao~ Paulo, Brazil; eCentre for Evolutionary Biology and Biodiversity, The University of Adelaide, Adelaide, SA 5005, Australia; fGraduate School of Human and Environmental Studies, Kyoto University, Sakyo-ku, Kyoto 606-8501, Japan; gSystematics Lab, Department of Environmental Studies, University of Delhi, Delhi 110 007, India; hDepartment of Biological Science, Florida State University, Tallahassee, FL 32306, USA; iDepartment of Scientific Computing, Florida State University, Dirac Science Library, Tallahassee, FL 32306-4120, USA; jDivision of Invertebrate Zoology, American Museum of Natural History, Central Park West at 79th Street, New York, NY 10024, USA Accepted 4 February 2015 Abstract Despite considerable progress in unravelling the phylogenetic relationships of microhylid frogs, relationships among subfami- lies remain largely unstable and many genera are not demonstrably monophyletic. -
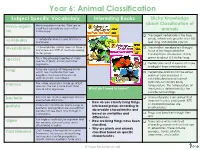
Animal Classification Subject Specific Vocabulary Interesting Books Sticky Knowledge About Classification of Micro-Organi Micro-Organisms Are Tiny
Year 6: Animal Classification Subject Specific Vocabulary Interesting Books Sticky Knowledge about Classification of micro-organi Micro-organisms are tiny. They are so small they can only be seen with a animals sm microscope. ❑ The largest vertebrate is the blue A vertebrate animal is one that has a whale, which can grow to over 100 vertebrates backbone. feet long and 400,000 pounds. An Invertebrate animal does not have a invertebrates ❑ The smallest vertebrate is thought backbone and 97% of creatures belong to be a tiny frog called the to this group. Paedophryne amauensis. It only This is the grouping together of similar grows to about 0.3 inches long. species species of plant, animal and other organisms. ❑ Vertebrates tend to be much more intelligent than invertebrates. Fungi are a group of living organisms fungi which are classified in their own ❑ Vertebrate animals can be either kingdom. This means they are not warm or cold-blooded. A animals, plants, or bacteria. cold-blooded animal cannot The whole organism is made up of just maintain a constant body monera one cell. The cell is more basic than temperature. The temperature of cells of other organisms. What do I need to know? their body is determined by the outside surroundings. Bacteria are tiny little organisms that are An invertebrate is an animal that bacteria ❑ everywhere around us. • How do you classify living things does not have a backbone. 97% Protists are not animals, plants, fungi, or of all animal species are protista into broad groups according to bacteria. Many protists are so small that invertebrates. -

Polyploidy and Sex Chromosome Evolution in Amphibians
Chapter 18 Polyploidization and Sex Chromosome Evolution in Amphibians Ben J. Evans, R. Alexander Pyron and John J. Wiens Abstract Genome duplication, including polyploid speciation and spontaneous polyploidy in diploid species, occurs more frequently in amphibians than mammals. One possible explanation is that some amphibians, unlike almost all mammals, have young sex chromosomes that carry a similar suite of genes (apart from the genetic trigger for sex determination). These species potentially can experience genome duplication without disrupting dosage stoichiometry between interacting proteins encoded by genes on the sex chromosomes and autosomalPROOF chromosomes. To explore this possibility, we performed a permutation aimed at testing whether amphibian species that experienced polyploid speciation or spontaneous polyploidy have younger sex chromosomes than other amphibians. While the most conservative permutation was not significant, the frog genera Xenopus and Leiopelma provide anecdotal support for a negative correlation between the age of sex chromosomes and a species’ propensity to undergo genome duplication. This study also points to more frequent turnover of sex chromosomes than previously proposed, and suggests a lack of statistical support for male versus female heterogamy in the most recent common ancestors of frogs, salamanders, and amphibians in general. Future advances in genomics undoubtedly will further illuminate the relationship between amphibian sex chromosome degeneration and genome duplication. B. J. Evans (CORRECTED&) Department of Biology, McMaster University, Life Sciences Building Room 328, 1280 Main Street West, Hamilton, ON L8S 4K1, Canada e-mail: [email protected] R. Alexander Pyron Department of Biological Sciences, The George Washington University, 2023 G St. NW, Washington, DC 20052, USA J. -

Conservation of Herpetofauna in Bantimurung Bulusaraung National Park, South Sulawesi, Indonesia
CONSERVATION OF HERPETOFAUNA IN BANTIMURUNG BULUSARAUNG NATIONAL PARK, SOUTH SULAWESI, INDONESIA Final Report 2008 By: M. Irfansyah Lubis, Wempy Endarwin, Septiantina D. Riendriasari, Suwardiansah, Adininggar U. Ul-Hasanah, Feri Irawan, Hadijah Aziz K., and Akmal Malawi Departemen Konservasi Sumberdaya Hutan Fakultas Kehutanan Institut Pertanian Bogor Bogor Indonesia 16000 Tel : +62 – 251 – 621 947 Fax: +62 – 251 – 621 947 Email: [email protected] (team leader) Conservation of Herpetofauna in Bantimurung Bulusaraung National Park, South Sulawesi, Indonesia Executive Summary Sulawesi is an island with complex geological and geographical history, thus resulting in a complex array in biodiversity. Bantimurung Bulusaraung National Park (BabulNP) was gazetted in 2004 to protect the region’s biodiversity and karst ecosystem. However, the park’s herpetofauna is almost unknown. This project consists of three programs: herpetofauna survey in BabulNP, herpetofauna conservation education to local schools, and herpetofauna training for locals and was conducted from July to September 2007. Based on the survey conducted in six sites in the park, we recorded 12 amphibian and 25 reptile species. Five of those species (Bufo celebensis, Rana celebensis, Rhacophorus monticola, Sphenomorphus tropidonotus, and Calamaria muelleri) are endemic to Sulawesi. Two species of the genus Oreophryne are still unidentified. We visited six schools around the park for our herpetofauna conservation education program. The Herpetofauna Observation Training was held over four days with 17 participants from BabulNP staff, local NGOs, school teachers, and Hasanuddin University students. i Conservation of Herpetofauna in Bantimurung Bulusaraung National Park, South Sulawesi, Indonesia Acknowledgements This project would not have been possible without the contribution of many persons. We would like to express our gratitude to BP Conservation Leadership Programme for providing funding. -

View Preprint
A peer-reviewed version of this preprint was published in PeerJ on 30 March 2017. View the peer-reviewed version (peerj.com/articles/3077), which is the preferred citable publication unless you specifically need to cite this preprint. Oliver PM, Iannella A, Richards SJ, Lee MSY. 2017. Mountain colonisation, miniaturisation and ecological evolution in a radiation of direct-developing New Guinea Frogs (Choerophryne, Microhylidae) PeerJ 5:e3077 https://doi.org/10.7717/peerj.3077 Mountain colonisation, miniaturisation and ecological evolution in a radiation of direct developing New Guinea Frogs (Choerophryne, Microhylidae) Paul M Oliver Corresp., 1 , Amy Iannella 2 , Stephen J Richards 3 , Michael S.Y Lee 3, 4 1 Division of Ecology and Evolution, Research School of Biology & Centre for Biodiversity Analysis, Australian National University, Canberra, Australian Capital Territory, Australia 2 School of Biological Sciences, The University of Adelaide, Adelaide, South Australia, Australia 3 South Australian Museum, Adelaide, South Australia, Australia 4 School of Biological Sciences, Flinders University, Adelaide, South Australia, Australia Corresponding Author: Paul M Oliver Email address: [email protected] Aims. Mountain ranges in the tropics are characterised by high levels of localised endemism, often-aberrant evolutionary trajectories, and some of the world’s most diverse regional biotas. Here we investigate the evolution of montane endemism, ecology and body size in a clade of direct-developing frogs (Choerophryne, Microhylidae) from New Guinea. Methods. Phylogenetic relationships were estimated from a mitochondrial molecular dataset using Bayesian and maximum likelihood approaches. Ancestral state reconstruction was used to infer the evolution of elevational distribution, ecology (indexed by male calling height), and body size, and phylogenetically corrected regression was employed to examine the relationships between these three traits. -

Two New Frog Species from the Foja Mountains in Northwestern New Guinea (Amphibia, Anura, Microhylidae)
68 (2): 109 –122 © Senckenberg Gesellschaft für Naturforschung, 2018. 28.5.2018 Two new frog species from the Foja Mountains in north western New Guinea (Amphibia, Anura, Micro hylidae) Rainer Günther 1, Stephen Richards 2 & Burhan Tjaturadi 3 1 Museum für Naturkunde, Invalidenstr. 43, 10115 Berlin, Germany; [email protected] — 2 Herpetology Department, South Australian Museum, North Terrace, Adelaide, South Australia 5000, Australia; [email protected] — 3 Conservation Inter- national – Papua Program. Current address: Center for Environmental Studies, Sanata Dharma University (CESSDU), Yogyakarta, Indonesia; [email protected] Accepted January 18, 2018. Published online at www.senckenberg.de/vertebrate-zoology on May 28, 2018. Editor in charge: Raffael Ernst Abstract Two new microhylid frogs in the genera Choerophryne and Oreophryne are described from the Foja Mountains in Papua Province of Indonesia. Both are small species (males 15.9 – 18.5 mm snout-urostyle length [SUL] and 21.3 – 22.9 mm SUL respectively) that call from elevated positions on foliage in primary lower montane rainforest. The new Choerophryne species can be distinguished from all congeners by, among other characters, a unique advertisement call consisting of an unpulsed (or very finely pulsed) peeping note last- ing 0.29 – 0.37 seconds. The new Oreophryne species belongs to a group that has a cartilaginous connection between the procoracoid and scapula and rattling advertisement calls. Its advertisement call is a loud rattle lasting 1.2 – 1.5 s with a note repetition rate of 11.3 – 11.7 notes per second. Kurzfassung Es werden zwei neue Engmaulfrösche der Gattungen Choerophryne und Oreophryne aus den Foja-Bergen in der Papua Provinz von Indonesien beschrieben. -

Anura:Microhylidae
THE PHYLOGENY OF THE PAPUAN SUBFAIÏILY ASTEROPHRYINAE (ANURA: MICROHYLIDAE) by THO¡1IAS CHARLES BURTON, 8.4., B.Sc. (Hons)MeIb. Department of Zoology University of Adelaide A thesis submitted to the UniVersity of Adelaide for the degree of Doctor of PhilosoPhY JT'NE 1.9 8 3 To ChwLø SUMMARY THE PHYLOGENY OF THE PAPUAN SUBFAMILY ASTEROPHRYINAE (nruunR : MI cRoHyt-rrRE) The Asterophryinae is a subfamily of terrestrial and fossorial microhylid frogs restricted to the Papuan Sub- Region. It comprises 43 named species and subspecies in seven genera. A second microhylid subfamily, the Sphenophryninae, also occurs in the Papuan Sub-Region, and its relationship to the Asterophryinae is contentious- In this study I undertake a phylogenetic analysis of the Asterophryinae based on the results of an examination of the myology, osteology and external morphology of members of all of the genera, and also of members of the Sphenophryninae, other microhylid. subfamilies and the Ranoidea, which serve as out-groups at different levels of analysis. The Asterophryinae and Sphenophryninae form a monophyletic group (sensu Hennig, 19661 supported by two autapomorphies: (a) direct embryonic development within the egg capsule; and (b) fusion and enlargement of the palatine and prevomer. The monophyly of the Asterophryinae is supported by three autapomorphies: (a) posterior adherence of the tongue and its division into anterior and posterior sections; (b) fusion of elements of the mandible and displacement of the mentomeckelians from the anterior margin of the mandible; and (c) loss of a dorsal el-ement J-aa of the M. intermandíbuLaris. The monophyly of the Sphenophryninae is supported by only one character of dubious value: procoely of the vertebral column. -

ARAZPA Amphibian Action Plan
Appendix 1 to Murray, K., Skerratt, L., Marantelli, G., Berger, L., Hunter, D., Mahony, M. and Hines, H. 2011. Guidelines for minimising disease risks associated with captive breeding, raising and restocking programs for Australian frogs. A report for the Australian Government Department of Sustainability, Environment, Water, Population and Communities. ARAZPA Amphibian Action Plan Compiled by: Graeme Gillespie, Director Wildlife Conservation and Science, Zoos Victoria; Russel Traher, Amphibian TAG Convenor, Curator Healesville Sanctuary Chris Banks, Wildlife Conservation and Science, Zoos Victoria. February 2007 1 1. Background Amphibian species across the world have declined at an alarming rate in recent decades. According to the IUCN at least 122 species have gone extinct since 1980 and nearly one third of the world’s near 6,000 amphibian species are classified as threatened with extinction, placing the entire class at the core of the current biodiversity crisis (IUCN, 2006). Australasia too has experienced significant declines; several Australian species are considered extinct and nearly 25% of the remainder are threatened with extinction, while all four species native to New Zealand are threatened. Conventional causes of biodiversity loss, habitat destruction and invasive species, are playing a major role in these declines. However, emergent disease and climate change are strongly implicated in many declines and extinctions. These factors are now acting globally, rapidly and, most disturbingly, in protected and near pristine areas. Whilst habitat conservation and mitigation of threats in situ are essential, for many taxa the requirement for some sort of ex situ intervention is mounting. In response to this crisis there have been a series of meetings organised by the IUCN (World Conservation Union), WAZA (World Association of Zoos & Aquariums) and CBSG (Conservation Breeding Specialist Group, of the IUCN Species Survival Commission) around the world to discuss how the zoo community can and should respond. -

Rampant Tooth Loss Across 200 Million Years of Frog Evolution
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429809; this version posted February 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Rampant tooth loss across 200 million years of frog evolution 2 3 4 Daniel J. Paluh1,2, Karina Riddell1, Catherine M. Early1,3, Maggie M. Hantak1, Gregory F.M. 5 Jongsma1,2, Rachel M. Keeffe1,2, Fernanda Magalhães Silva1,4, Stuart V. Nielsen1, María Camila 6 Vallejo-Pareja1,2, Edward L. Stanley1, David C. Blackburn1 7 8 1Department of Natural History, Florida Museum of Natural History, University of Florida, 9 Gainesville, Florida USA 32611 10 2Department of Biology, University of Florida, Gainesville, Florida USA 32611 11 3Biology Department, Science Museum of Minnesota, Saint Paul, Minnesota USA 55102 12 4Programa de Pós Graduação em Zoologia, Universidade Federal do Pará/Museu Paraense 13 Emilio Goeldi, Belém, Pará Brazil 14 15 *Corresponding author: Daniel J. Paluh, [email protected], +1 814-602-3764 16 17 Key words: Anura; teeth; edentulism; toothlessness; trait lability; comparative methods 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429809; this version posted February 6, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. -

Occasional Papers of the Museum of Zoology University of Michigan
NUMBER 745 AUGUST 20, 2015 OCCASIONAL PAPERS OF THE MUSEUM OF ZOOLOGY UNIVERSITY OF MICHIGAN ANN ARBOR, MICHIGAN A NEW SPECIES OF THE MINIATURIZED FROG GENUS PAEDOPHRYNE (ANURA: MICROHYLIDAE) FROM PAPUA NEW GUINEA Fred Kraus1 ABSTRACT – I describe a new species of Paedophryne from low-elevation foothill rainforest on Normanby Island off southeastern Papua New Guinea. This new species is the largest member of the genus so far discovered, with body length of the sole adult male 11.1 mm, and with adult females ranging from 10.6–11.6 mm. As well, it differs from other species in the genus in having smooth dorsal skin, a disc on the second toe, relatively larger first digits, and in a number of body proportions and details of color pattern. As for other species in the genus, it is an inhabitant of rainforest leaf litter restricted to a portion of the East Papuan Composite Terrane, an agglomeration of terranes that were assembled offshore of New Guinea and sutured to it during the early Miocene. Key words: D’Entrecasteaux Islands; East Papuan Composite Terrane, Milne Bay Province; Normanby Island INTRODUCTION The most miniaturized lineage of tetrapods is Paedophryne, a Papuan genus of microhylid frog only recently described (Kraus, 2010) and now known from six species (Kraus, 2011; Rittmeyer et al., 2012). Adults of these species vary from 7.0–11.3 mm snout-vent length, with females being slightly larger than males, as is typical for Papuan microhylids. All species are inhabitants of the interstices within leaf litter and have a unique combination of morphological characters.