For Peer Review Only
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Recent Changes in the Names of New Zealand Tree and Shrub Species
-- -- - Recent changes in the names of New Zealand tree and shrub species - Since the publication of 'Flora of New Zealand' Volume 1 (A- iii) Podocarpus dacydioides Dacrycarpus ducydioides lan 1961),covering indigenous ferns, conifers and dicots, there (iii)Podocarpus ferrugzneus Prumnopitys ferruginea have been major advances in taxonomic research and the clas- Podocarpus spicatus Prumnopitys taxijolia sification of many plant groups revised accordingly. Most of (iv1 Dacrydium cupressinum (unchanged) these changes have been summarised in the Nomina Nova (v)Dacrydium bidwillii Halocarpus bidwillii series published in the New Zealand Journal of Botany (Edgar Dacrydium bijorme Halocarpus bijormis 1971, Edgar and Connor 1978, 1983) and are included in re- Dacrydium kirkii Halocarpus kirkii cent books on New Zealand plants ie.g. Eagle 1982, Wilson (vi)Dacydium colensoi Lagarostrobos colensoi 1982). A number of these name changes affect important (vii)Dacrydium intermediurn Lepidothamnus intermedius forest plants and as several of these new names are now start- Dacrydium laxijolium Lepidotbamnus laxijolius ing to appear in the scientific literature, a list of changes af- (viii)Phyllocladus trichomanoidi~(unchanged) fecting tree and shrub taxa are given here. As a large number Phyllocladus glaucus (unchanged) of the readers of New Zealand Forestry are likely to use Poole Phyllocladus alpinus Phyllocladus aspleniijolius and Adams' "Trees and Shrubs of New Zealand" as their var. alpinus* * main reference for New Zealand forest plants, all the name changes are related to the fourth impression of this book. * It has been suggested that the Colenso name P, cunnin- it is important to realise that not all botanists necessarily ghamii (1884)should take precedence over the later (18891 ark agree with one particular name and you are not obliged to use name (P. -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Re-Establishing North Island Kākā (Nestor Meridionalis Septentrionalis
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Re-establishing North Island kākā (Nestor meridionalis septentrionalis) in New Zealand A thesis presented in fulfilment of the requirements for the degree of Master of Science In Conservation Biology Massey University Auckland, New Zealand Tineke Joustra 2018 ii For Orlando, Aurora and Nayeli “I don’t want my children to follow in my footsteps, I want them to take the path next to me and go further than I could have ever dreamt possible” Anonymous iii iv Abstract Recently there has been a global increase in concern over the unprecedented loss of biodiversity and how the sixth mass extinction event is mainly due to human activities. Countries such as New Zealand have unique ecosystems which led to the evolution of many endemic species. One such New Zealand species is the kākā (Nestor meridionalis). Historically, kākā abundance has been affected by human activities (kākā were an important food source for Māori and Europeans). Today, introduced mammalian predators are one of the main threats to wild kākā populations. Although widespread and common throughout New Zealand until the 1800’s, kākā populations on the mainland now heavily rely on active conservation management. The main methods of kākā management include pest control and re-establishments. This thesis evaluated current and past commitments to New Zealand species restoration, as well as an analysis of global Psittacine re-establishment efforts. -

Exploring the Source-To-Sink Residence Time of Terrestrial Pollen Deposited Offshore Westland, New Zealand
ÔØ ÅÒÙ×Ö ÔØ Exploring the source-to-sink residence time of terrestrial pollen deposited offshore Westland, New Zealand Matthew T. Ryan, Rewi M. Newnham, Gavin B. Dunbar, Marcus J. Vandergoes, Andrew B.H. Rees, Helen Neil, S. Louise Callard, Brent V. Alloway, Helen Bostock, Quan Hua, Brian M. Anderson PII: S0034-6667(15)30011-7 DOI: doi: 10.1016/j.revpalbo.2016.03.005 Reference: PALBO 3738 To appear in: Review of Palaeobotany and Palynology Received date: 14 September 2015 Accepted date: 26 March 2016 Please cite this article as: Ryan, Matthew T., Newnham, Rewi M., Dunbar, Gavin B., Vandergoes, Marcus J., Rees, Andrew B.H., Neil, Helen, Callard, S. Louise, Alloway, Brent V., Bostock, Helen, Hua, Quan, Anderson, Brian M., Exploring the source-to-sink residence time of terrestrial pollen deposited offshore Westland, New Zealand, Review of Palaeobotany and Palynology (2016), doi: 10.1016/j.revpalbo.2016.03.005 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT Exploring the source-to-sink residence time of terrestrial pollen deposited offshore Westland, New Zealand Matthew T. Ryan1, 2, Rewi M. Newnham1, Gavin B. Dunbar 2, Marcus J. Vandergoes 3, Andrew B.H. -

Ecology and Distribution of the Malesian Podocarps Neal J
4 Ecology and Distribution of the Malesian Podocarps Neal J. Enright and Tanguy Jaffré ABSTRACT. Podocarp species and genus richness is higher in the Malesian region than anywhere else on earth, with maximum genus richness in New Guinea and New Caledo- nia and maximum species richness in New Guinea and Borneo. Members of the Podo- carpaceae occur across the whole geographic and altitudinal range occupied by forests and shrublands in the region. There is a strong tendency for podocarp dominance of vegetation to be restricted either to high- altitude sites close to the limit of tree growth or to other sites that might restrict plant growth in terms of water relations and nutri- ent supply (e.g., skeletal soils on steep slopes and ridges, heath forests, ultramafic parent material). Although some species are widespread in lowland forests, they are generally present at very low density, raising questions concerning their regeneration ecology and competitive ability relative to co- occurring angiosperm tree species. A number of species in the region are narrowly distributed, being restricted to single islands or mountain tops, and are of conservation concern. Our current understanding of the distribution and ecology of Malesian podocarps is reviewed in this chapter, and areas for further research are identified. INTRODUCTION The Malesian region has the highest diversity of southern conifers (i.e., Podocarpaceae and Araucariaceae) in the world (Enright and Hill, 1995). It is a large and heterogeneous area, circumscribing tropical and subtropical lowland to montane forest (and some shrubland) assemblages, extending from Tonga in Neal J. Enright, School of Environmental Science, the east to India in the west and from the subtropical forests of eastern Australia Murdoch University, Murdoch, Western Austra- in the south to Taiwan and Nepal in the north (Figure 4.1). -
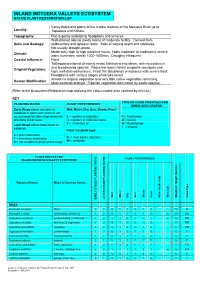
Inland Motueka Valleys Plant Lists
INLAND MOTUEKA VALLEYS ECOSYSTEM NATIVE PLANT RESTORATION LIST Valley floors and plains of the middle reaches of the Motueka River up to Locality: Tapawera and Kohatu. Topography: Flat to gently undulating floodplains and terraces. Well-drained alluvial sandy loams of moderate fertility. Derived from Soils and Geology: sedimentary and igneous rocks. Soils of varying depth and stoniness. Not usually drought-prone. Moderately high to high sunshine hours; frosts moderate to moderately severe; Climate: warm summers; rainfall 1200-1600mm. Droughts infrequent. Coastal influence: None Tall podocarp forest of mainly mataī, kāhikatea and tōtara, with mixed beech and broadleaved species. Red-silver beech forest on gentle toeslopes and Original Vegetation: high, well-drained terraces. Frost flat shrublands on terraces with severe frost. Floodplains with various stages of kānuka forest. Almost no original vegetation and very little native vegetation remaining. Human Modification: Most wetlands drained. Riparian vegetation dominated by exotic species. [Refer to the Ecosystem Restoration map showing the colour-coded area covered by this list.] KEY TYPE OF FOOD PROVIDED FOR PLANTING RATIO PLANT PREFERENCES BIRDS AND LIZARDS Early Stage plants are able to Wet, Moist, Dry, Sun, Shade, Frost establish in open sites and can act as a nursery for later stage plants by 1 = prefers or tolerates F = Fruit/seeds providing initial cover. ½ = prefers or tolerates some N = Nectar 0 = intolerant of B = Buds/foliage Later Stage plants need cover to I = Insects establish. -

The Evolution of Cavitation Resistance in Conifers Maximilian Larter
The evolution of cavitation resistance in conifers Maximilian Larter To cite this version: Maximilian Larter. The evolution of cavitation resistance in conifers. Bioclimatology. Univer- sit´ede Bordeaux, 2016. English. <NNT : 2016BORD0103>. <tel-01375936> HAL Id: tel-01375936 https://tel.archives-ouvertes.fr/tel-01375936 Submitted on 3 Oct 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. THESE Pour obtenir le grade de DOCTEUR DE L’UNIVERSITE DE BORDEAUX Spécialité : Ecologie évolutive, fonctionnelle et des communautés Ecole doctorale: Sciences et Environnements Evolution de la résistance à la cavitation chez les conifères The evolution of cavitation resistance in conifers Maximilian LARTER Directeur : Sylvain DELZON (DR INRA) Co-Directeur : Jean-Christophe DOMEC (Professeur, BSA) Soutenue le 22/07/2016 Devant le jury composé de : Rapporteurs : Mme Amy ZANNE, Prof., George Washington University Mr Jordi MARTINEZ VILALTA, Prof., Universitat Autonoma de Barcelona Examinateurs : Mme Lisa WINGATE, CR INRA, UMR ISPA, Bordeaux Mr Jérôme CHAVE, DR CNRS, UMR EDB, Toulouse i ii Abstract Title: The evolution of cavitation resistance in conifers Abstract Forests worldwide are at increased risk of widespread mortality due to intense drought under current and future climate change. -

Tree Holes in a Mixed Broad-Leaf–Podocarp Rain Forest, New Zealand
BLAKELY,Available on-line DIDHAM: at: http://www.newzealandecology.org/nzje/ TREE HOLES IN TEMPERATE RAIN FOREST 197 Tree holes in a mixed broad-leaf–podocarp rain forest, New Zealand Tanya J. Blakely* and Raphael K. Didham School of Biological Sciences, University of Canterbury, Private Bag 4800, Christchurch 8140, New Zealand *Author for correspondence (Email: [email protected]) Published on-line: 27 August 2008 ___________________________________________________________________________________________________________________________________ Abstract: Despite the ecological importance of tree holes as habitat for many species in New Zealand, few studies have quantified the abundance, distribution or structural characteristics of tree holes in native forests. We recorded a total of 364 tree holes in ground-to-canopy surveys on 50 trees of five endemic species in the families Fagaceae and Podocarpaceae within Orikaka Ecological Area, Buller District, New Zealand. Tree holes were not uniformly distributed throughout the forest, with more holes in the three podocarp species Prumnopitys ferruginea, P. taxifolia and Dacrycarpus dacrydioides than in Nothofagus fusca or N. menziesii. However, N. fusca had the largest tree holes of any of the tree species sampled. Tree-hole volume and tree-hole opening both increased with tree size. Tree-hole opening was strongly positively correlated with internal volume, especially for the podocarps. It therefore potentially provides a useful surrogate measure for quantifying the abundance of large tree holes from cost-effective ground surveys. We estimated there might be as many as 771 tree holes per hectare of forest at this site, but fewer of these were thought to be large enough for obligate hole-dwelling vertebrate species. -

Structure and Canopy Tree Species Regeneration Requirements in Indigenous Forests, Westland, New Zealand
Structure and canopy tree species regeneration requirements in indigenous forests, Westland, New Zealand DOC SCIENCE INTERNAL SERIES 66 Glenn H. Stewart Published by Department of Conservation P.O. Box 10-420 Wellington, New Zealand DOC Science Internal Series is a published record of scientific research carried out, or advice given, by Department of Conservation staff, or external contractors funded by DOC. It comprises progress reports and short communications that are generally peer-reviewed within DOC, but not always externally refereed. Fully refereed contract reports funded from the Conservation Services Levy are also included. Individual contributions to the series are first released on the departmental intranet in pdf form. Hardcopy is printed, bound, and distributed at regular intervals. Titles are listed in the DOC Science Publishing catalogue on the departmental website http://www.doc.govt.nz and electronic copies of CSL papers can be downloaded from http://csl.doc.govt.nz © August 2002, New Zealand Department of Conservation ISSN 1175–6519 ISBN 0–478–22299–8 This is a client report commissioned by West Coast Conservancy and funded from the Unprogrammed Science Advice fund . It was prepared for publication by DOC Science Publishing, Science & Research Unit; editing and layout by Geoff Gregory. Publication was approved by the Manager, Science & Research Unit, Science Technology and Information Services, Department of Conservation, Wellington. CONTENTS Abstract 5 1. Introduction 6 2. Review of relevant ecological literature 7 2.1 North Westland 7 2.1.1 Inland valleys 7 2.1.2 Western valleys 12 2.1.3 Coastal forests 17 2.2 Central Westland 17 2.2.1 Inland valleys 17 2.2.2 Coastal forests 18 2.3 South Westland 18 2.3.1 Inland valleys 18 2.3.2 Coastal outwash surfaces 19 3. -

11 Podocarp Roots, Mycorrhizas, and Nodules Ian A
11 Podocarp Roots, Mycorrhizas, and Nodules Ian A. Dickie and Robert J. Holdaway ABSTRACT. Podocarps are often found on low- nutrient soils and further exacerbate nutrient limitation through the production of recalcitrant litter. Understanding podo- carp ecology therefore depends on understanding the root adaptations of podocarps to obtain nutrients. The roots of podocarps are heterorhizic (having two root forms) with abundant spherical determinate short roots, a unique form of largely unknown function, as well as indeterminate fine roots. There is no strong evidence for nitrogen fixation in the nodules. Although nodules are frequently and abundantly colonized by arbuscular mycorrhizal fungi, they are neither required for mycorrhizal infection nor dependent on mycorrhizal infection for their development. The fungal symbiont community associated with podocarps does not appear to be distinctive from that of other plants at a global scale, although it may differ from co-occurring plants within a site. Perhaps the most obvious, yet largely overlooked, hypothesis is that the form of the nodules is the func- tion. The presence of nodules permits a doubling of root cortex volume, while simple geometry indicates that a spherical form involves the lowest possible cost in producing cell wall and membrane material per unit of root volume. Increased root volume permits greater arbuscular mycorrhizal interaction, and hence, nodules may be analogous to the determinate ectomycorrhizal roots of the Pinaceae as a structure to maximize fungal interactions at minimal root construction cost. INTRODUCTION Ian A. Dickie, Landcare Research, Box 40, Lin- Podocarps (gymnosperms in the Podocarpaceae) grow on a range of soils coln 7640, New Zealand. -

Sanctuary Mountain Maungatautari Restoration Plan 2019–2029
Sanctuary Mountain Maungatautari Restoration Plan 2019–2029 Prepared for: Maungatautari Ecological Island Trust June 2019 Sanctuary Mountain Maungatautari Restoration Plan 2019–2029 Contract Report: LC3464 John Innes, Corinne Watts Manaaki Whenua – Landcare Research Bruce Burns University of Auckland With contributions from other members of, and advisors to, the Maungatautari Scientific and Technical Advisory Panel: Aaron Barnsdall (Mana Whenua Trustee) Geoff Churchill (Maungatautari Ecological Island Trust) Professor Bruce Clarkson (University of Waikato) Poto Davies (Maungatautari Mana Whenua) Dr Catherine Kirby (University of Waikato) Cheridan Mathers (Hamilton Zoo) Dr Shelley Langton-Myers (EcoQuest Education Foundation) Robyn Nightingale (Maungatautari Mana Whenua) Dr Kate Richardson (Waikato Regional Council) Tony Roxburgh (Waipa District Council) Alan Saunders (Waikato Regional Council) Dr Andrew Styche (Department of Conservation) Dr Kiri Wallace (University of Waikato) Dr Janelle Ward (Maungatautari Ecological Island Trust) Reviewed by: Approved for release by: Robyn Simcock Gary Houliston Scientist Portfolio Leader – Enhancing Biodiversity Manaaki Whenua – Landcare Research Manaaki Whenua – Landcare Research Disclaimer This report has been prepared by Manaaki Whenua – Landcare Research for Maungatautari Ecological Island Trust. If used by other parties, no warranty or representation is given as to its accuracy and no liability is accepted for loss or damage arising directly or indirectly from reliance on the information in it. -

Download Article As 724.4 KB PDF File
66 AvailableNew on-lineZealand at: Journal http://www.newzealandecology.org/nzje/ of Ecology, Vol. 34, No. 1, 2010 special issue: Feathers to Fur The ecological transformation of Aotearoa/New Zealand Mutualisms with the wreckage of an avifauna: the status of bird pollination and fruit- dispersal in New Zealand Dave Kelly1*, Jenny J. Ladley1, Alastair W. Robertson2, Sandra H. Anderson3, Debra M. Wotton1, and Susan K. Wiser4 1School of Biological Sciences, University of Canterbury, Private Bag 4800, Christchurch 8140, New Zealand 2Ecology, Institute of Natural Resources, Massey University, Private Bag 11222, Palmerston North 4474, New Zealand 3School of Environment, University of Auckland, Private Bag 92019, Auckland 1010, New Zealand 4Landcare Research, PO Box 40, Lincoln 7640, New Zealand *Author for correspondence (Email: [email protected]) Published on-line: 9 November 2009 Abstract: Worldwide declines in bird numbers have recently renewed interest in how well bird–plant mutualisms are functioning. In New Zealand, it has been argued that bird pollination was relatively unimportant and bird- pollination failure was unlikely to threaten any New Zealand plants, whereas dispersal mutualisms were widespread and in some cases potentially at risk because of reliance on a single large frugivore, the kereru (Hemiphaga novaeseelandiae). Work since 1989, however, has changed that assessment. Smaller individual fruits of most plant species can be dispersed by mid-sized birds such as tui (Prosthemadera novaezelandiae) because both fruits and birds vary in size within a species. Only one species (Beilschmiedia tarairi) has no individual fruits small enough for this to occur. Germination of 19 fleshy-fruited species, including most species with fruits >8 mm diameter, does not depend on birds removing the fruit pulp.