Structure and Canopy Tree Species Regeneration Requirements in Indigenous Forests, Westland, New Zealand
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recent Changes in the Names of New Zealand Tree and Shrub Species
-- -- - Recent changes in the names of New Zealand tree and shrub species - Since the publication of 'Flora of New Zealand' Volume 1 (A- iii) Podocarpus dacydioides Dacrycarpus ducydioides lan 1961),covering indigenous ferns, conifers and dicots, there (iii)Podocarpus ferrugzneus Prumnopitys ferruginea have been major advances in taxonomic research and the clas- Podocarpus spicatus Prumnopitys taxijolia sification of many plant groups revised accordingly. Most of (iv1 Dacrydium cupressinum (unchanged) these changes have been summarised in the Nomina Nova (v)Dacrydium bidwillii Halocarpus bidwillii series published in the New Zealand Journal of Botany (Edgar Dacrydium bijorme Halocarpus bijormis 1971, Edgar and Connor 1978, 1983) and are included in re- Dacrydium kirkii Halocarpus kirkii cent books on New Zealand plants ie.g. Eagle 1982, Wilson (vi)Dacydium colensoi Lagarostrobos colensoi 1982). A number of these name changes affect important (vii)Dacrydium intermediurn Lepidothamnus intermedius forest plants and as several of these new names are now start- Dacrydium laxijolium Lepidotbamnus laxijolius ing to appear in the scientific literature, a list of changes af- (viii)Phyllocladus trichomanoidi~(unchanged) fecting tree and shrub taxa are given here. As a large number Phyllocladus glaucus (unchanged) of the readers of New Zealand Forestry are likely to use Poole Phyllocladus alpinus Phyllocladus aspleniijolius and Adams' "Trees and Shrubs of New Zealand" as their var. alpinus* * main reference for New Zealand forest plants, all the name changes are related to the fourth impression of this book. * It has been suggested that the Colenso name P, cunnin- it is important to realise that not all botanists necessarily ghamii (1884)should take precedence over the later (18891 ark agree with one particular name and you are not obliged to use name (P. -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Pollination Drop in Relation to Cone Morphology in Podocarpaceae: a Novel Reproductive Mechanism Author(S): P
Pollination Drop in Relation to Cone Morphology in Podocarpaceae: A Novel Reproductive Mechanism Author(s): P. B. Tomlinson, J. E. Braggins, J. A. Rattenbury Source: American Journal of Botany, Vol. 78, No. 9 (Sep., 1991), pp. 1289-1303 Published by: Botanical Society of America Stable URL: http://www.jstor.org/stable/2444932 . Accessed: 23/08/2011 15:47 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Botanical Society of America is collaborating with JSTOR to digitize, preserve and extend access to American Journal of Botany. http://www.jstor.org AmericanJournal of Botany 78(9): 1289-1303. 1991. POLLINATION DROP IN RELATION TO CONE MORPHOLOGY IN PODOCARPACEAE: A NOVEL REPRODUCTIVE MECHANISM' P. B. TOMLINSON,2'4 J. E. BRAGGINS,3 AND J. A. RATTENBURY3 2HarvardForest, Petersham, Massachusetts 01366; and 3Departmentof Botany, University of Auckland, Auckland, New Zealand Observationof ovulatecones at thetime of pollinationin the southernconiferous family Podocarpaceaedemonstrates a distinctivemethod of pollencapture, involving an extended pollinationdrop. Ovules in all generaof the family are orthotropousand singlewithin the axil of each fertilebract. In Microstrobusand Phyllocladusovules are-erect (i.e., the micropyle directedaway from the cone axis) and are notassociated with an ovule-supportingstructure (epimatium).Pollen in thesetwo genera must land directly on thepollination drop in theway usualfor gymnosperms, as observed in Phyllocladus.In all othergenera, the ovule is inverted (i.e., the micropyleis directedtoward the cone axis) and supportedby a specializedovule- supportingstructure (epimatium). -

Re-Establishing North Island Kākā (Nestor Meridionalis Septentrionalis
Copyright is owned by the Author of the thesis. Permission is given for a copy to be downloaded by an individual for the purpose of research and private study only. The thesis may not be reproduced elsewhere without the permission of the Author. Re-establishing North Island kākā (Nestor meridionalis septentrionalis) in New Zealand A thesis presented in fulfilment of the requirements for the degree of Master of Science In Conservation Biology Massey University Auckland, New Zealand Tineke Joustra 2018 ii For Orlando, Aurora and Nayeli “I don’t want my children to follow in my footsteps, I want them to take the path next to me and go further than I could have ever dreamt possible” Anonymous iii iv Abstract Recently there has been a global increase in concern over the unprecedented loss of biodiversity and how the sixth mass extinction event is mainly due to human activities. Countries such as New Zealand have unique ecosystems which led to the evolution of many endemic species. One such New Zealand species is the kākā (Nestor meridionalis). Historically, kākā abundance has been affected by human activities (kākā were an important food source for Māori and Europeans). Today, introduced mammalian predators are one of the main threats to wild kākā populations. Although widespread and common throughout New Zealand until the 1800’s, kākā populations on the mainland now heavily rely on active conservation management. The main methods of kākā management include pest control and re-establishments. This thesis evaluated current and past commitments to New Zealand species restoration, as well as an analysis of global Psittacine re-establishment efforts. -

Beetles in a Suburban Environment: a New Zealand Case Study. The
tl n brbn nvrnnt: lnd td tl n brbn nvrnnt: lnd td h Idntt nd tt f Clptr n th ntrl nd dfd hbtt f nfld Alnd (4-8 GKhl . : rh At SI lnt rttn Mnt Albrt rh Cntr rvt Alnd lnd • SI lnt rttn prt • EW EAA EAME O SCIEIIC A IUSIA ESEAC 199 O Ο Ν Ε W Ε Ν ttr Grnt rd Τ Ε Ρ Ο Ι Ο Τ ie wi e suo o a oey Sciece eseac Ga om e ew eaa oey Gas oa is suo is gaeuy ackowege Ρ EW EAA SI ' EAME O lnt SCIEIIC A rttn IUSIA Wāhn ESEAC Mn p Makig Sciece Wok o ew eaa KUSCE G eees i a suua eiome a ew eaa case suy e ieiy a saus o Coeoea i e aua a moiie aias o yie Aucka (197-199 / G Kusce — Aucka SI 199 (SI a oecio eo ISS 11-1 ; o3 IS -77-59- I ie II Seies UC 5957(93111 © Cow Coyig uise y SI a oecio M Ae eseac Cee iae ag Aucka ew eaa eceme 199 ie y Geea iig Seices eso ew eaa Etiam pristina in aua Asο i a aua seig summa securitas et futura sweet tranquility and nature ., OISIECE e oe-eeig emoyci eee ioycus uuus (ou o is aie ooca os kaikaea (acycaus acyioies om e yie eee suey aea Aucka ew eaa e wie gaues o e eee ae oe cuses a ass ees is eee as a eic saus o uike a o e uaaa (Seoo as ossi eiece sows a e weei gou was iig i uassic imes way ack i e ea o e iosaus a gymosems moe a 1 miio yeas ago OEWO As a small boy in the 1930s I used to collect butterflies on the South Downs in southern England. -

Examples of Totara Sapwood Resisting Attack by the Common Household Wood Borer (Anobium Punctatum)
EXAMPLES OF TOTARA SAPWOOD RESISTING ATTACK BY THE COMMON HOUSEHOLD WOOD BORER (ANOBIUM PUNCTATUM) A Report Prepared by Paul Quinlan for Tāne’s Tree Trust 31st January 2017 1 EXAMPLES OF TOTARA SAPWOOD RESISTING ATTACK BY THE COMMON HOUSEHOLD BORER (ANOBIUM PUNCTATUM) 31 January 2017 Paul Quinlan Paul Quinlan Landscape Architect Ltd. [email protected] PH.: (09) 4050052 A report prepared on behalf of the Northland Totara Working Group. Purpose of report. Presently, the NZ Standards NZS 3602 committee is undertaking a review of aspects of the New Zealand Building Code. To assist with that process, this report documents evidence that members of the Northland Totara Working Group can verify regarding examples where tōtara sapwood timber has shown resistance to the common household borer (Anobium punctatum). Disclaimer: In producing this report, reasonable care has been taken regarding the accuracy of the information presented. However, no guarantee as to the truth, accuracy or validity of any of the comments, implications, recommendations, findings or conclusions are made by the author, the Northland Totara Working Group, Tāne’s Tree Trust, or any other party. Therefore, neither the authors, nor any of the supporting organisations, shall not be liable for, or accept any responsibility for, any loss, damage or liability incurred as a result of direct or indirect result of any reliance by any person upon information or opinions or recommendations expressed in this work. Users of any of this information, whether contained or inferred, in or arising from this report do so at their own risk. 2 Table of Contents Table of Contents ................................................................................................................................... -

BIOSECURITY IMPLICATIONS of EXOTIC BEETLES ATTACKING TREES and SHRUBS in NEW ZEALAND E. G. BROCKERHOFF1,3 and J. BAIN2
Forestry 321 BIOSECURITY IMPLICATIONS OF EXOTIC BEETLES ATTACKING TREES AND SHRUBS IN NEW ZEALAND E. G. BROCKERHOFF1,3 and J. BAIN2 1Forest Research, PO Box 29237, Christchurch 2Forest Research, Private Bag 3020, Rotorua 3Author for correspondence ABSTRACT A survey of exotic beetles that attack trees or shrubs in New Zealand found 51 species of mainly Australian (58%) and European (25%) origin. In addition, three biological control agents have been released against woody adventive plant pests. The host range of most species is restricted to exotic crop and ornamental plants in New Zealand. Nine polyphagous borers sometimes attack dead wood of indigenous species, and at least one polyphagous root feeder may attack indigenous trees, but the ecological impact of these species on indigenous forests appears negligible. However, some of the wood and bark borers as well as several defoliators are important pests of exotic crop and amenity plants. Although this suggests that exotic phytophagous beetles pose a greater biosecurity threat to exotics than to indigenous species, a greater surveillance effort in New Zealand’s indigenous forests appears necessary to detect potentially harmful invasions. Keywords: invasion, Coleoptera, host range, insect, tree. INTRODUCTION The colonisation of New Zealand by Polynesians and Europeans has led to the accidental or intentional introduction of numerous exotic plants and animals. Today there are over 2000 plant, nearly 100 vertebrate and over 2000 invertebrate species that are naturalised exotics (Atkinson and Cameron 1993; Ministry for the Environment 1997). The impact of invasive plant pests and mammalian herbivores and predators on New Zealand’s ecosystems is well recognised (Atkinson and Cameron 1993). -

Exploring the Source-To-Sink Residence Time of Terrestrial Pollen Deposited Offshore Westland, New Zealand
ÔØ ÅÒÙ×Ö ÔØ Exploring the source-to-sink residence time of terrestrial pollen deposited offshore Westland, New Zealand Matthew T. Ryan, Rewi M. Newnham, Gavin B. Dunbar, Marcus J. Vandergoes, Andrew B.H. Rees, Helen Neil, S. Louise Callard, Brent V. Alloway, Helen Bostock, Quan Hua, Brian M. Anderson PII: S0034-6667(15)30011-7 DOI: doi: 10.1016/j.revpalbo.2016.03.005 Reference: PALBO 3738 To appear in: Review of Palaeobotany and Palynology Received date: 14 September 2015 Accepted date: 26 March 2016 Please cite this article as: Ryan, Matthew T., Newnham, Rewi M., Dunbar, Gavin B., Vandergoes, Marcus J., Rees, Andrew B.H., Neil, Helen, Callard, S. Louise, Alloway, Brent V., Bostock, Helen, Hua, Quan, Anderson, Brian M., Exploring the source-to-sink residence time of terrestrial pollen deposited offshore Westland, New Zealand, Review of Palaeobotany and Palynology (2016), doi: 10.1016/j.revpalbo.2016.03.005 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT Exploring the source-to-sink residence time of terrestrial pollen deposited offshore Westland, New Zealand Matthew T. Ryan1, 2, Rewi M. Newnham1, Gavin B. Dunbar 2, Marcus J. Vandergoes 3, Andrew B.H. -

Ecology and Distribution of the Malesian Podocarps Neal J
4 Ecology and Distribution of the Malesian Podocarps Neal J. Enright and Tanguy Jaffré ABSTRACT. Podocarp species and genus richness is higher in the Malesian region than anywhere else on earth, with maximum genus richness in New Guinea and New Caledo- nia and maximum species richness in New Guinea and Borneo. Members of the Podo- carpaceae occur across the whole geographic and altitudinal range occupied by forests and shrublands in the region. There is a strong tendency for podocarp dominance of vegetation to be restricted either to high- altitude sites close to the limit of tree growth or to other sites that might restrict plant growth in terms of water relations and nutri- ent supply (e.g., skeletal soils on steep slopes and ridges, heath forests, ultramafic parent material). Although some species are widespread in lowland forests, they are generally present at very low density, raising questions concerning their regeneration ecology and competitive ability relative to co- occurring angiosperm tree species. A number of species in the region are narrowly distributed, being restricted to single islands or mountain tops, and are of conservation concern. Our current understanding of the distribution and ecology of Malesian podocarps is reviewed in this chapter, and areas for further research are identified. INTRODUCTION The Malesian region has the highest diversity of southern conifers (i.e., Podocarpaceae and Araucariaceae) in the world (Enright and Hill, 1995). It is a large and heterogeneous area, circumscribing tropical and subtropical lowland to montane forest (and some shrubland) assemblages, extending from Tonga in Neal J. Enright, School of Environmental Science, the east to India in the west and from the subtropical forests of eastern Australia Murdoch University, Murdoch, Western Austra- in the south to Taiwan and Nepal in the north (Figure 4.1). -
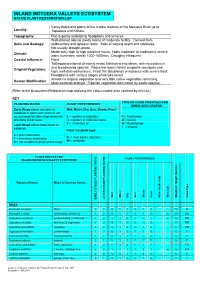
Inland Motueka Valleys Plant Lists
INLAND MOTUEKA VALLEYS ECOSYSTEM NATIVE PLANT RESTORATION LIST Valley floors and plains of the middle reaches of the Motueka River up to Locality: Tapawera and Kohatu. Topography: Flat to gently undulating floodplains and terraces. Well-drained alluvial sandy loams of moderate fertility. Derived from Soils and Geology: sedimentary and igneous rocks. Soils of varying depth and stoniness. Not usually drought-prone. Moderately high to high sunshine hours; frosts moderate to moderately severe; Climate: warm summers; rainfall 1200-1600mm. Droughts infrequent. Coastal influence: None Tall podocarp forest of mainly mataī, kāhikatea and tōtara, with mixed beech and broadleaved species. Red-silver beech forest on gentle toeslopes and Original Vegetation: high, well-drained terraces. Frost flat shrublands on terraces with severe frost. Floodplains with various stages of kānuka forest. Almost no original vegetation and very little native vegetation remaining. Human Modification: Most wetlands drained. Riparian vegetation dominated by exotic species. [Refer to the Ecosystem Restoration map showing the colour-coded area covered by this list.] KEY TYPE OF FOOD PROVIDED FOR PLANTING RATIO PLANT PREFERENCES BIRDS AND LIZARDS Early Stage plants are able to Wet, Moist, Dry, Sun, Shade, Frost establish in open sites and can act as a nursery for later stage plants by 1 = prefers or tolerates F = Fruit/seeds providing initial cover. ½ = prefers or tolerates some N = Nectar 0 = intolerant of B = Buds/foliage Later Stage plants need cover to I = Insects establish. -

Set 3 Plains Plant List AA
Food for native birds: HOUHERE – piwakawaka - kohuhu, F = Fruit S = Bird Seed N = Nectar mid age plains system B = Bud/foliage I = Insects For lizards: L = fruit Plant Tolerances ■ = tolerates or needs □ = intolerant ½ = tolerant of some * = to establish, protect from frost t = toxic for toddlers Staging PLANT LISTS Selected from vegetation natural to these moist & deep Waimakariri 1 = 1st structural 2 = 2nd year soils 3 = only after canopy closure Tolerances TALL (NOBLE) TREES (> 10 m) Food sun shade wet dry wind Stages Cordyline australis ti kouka, cabbage tree F,N,I ■ ½ ■ ■ ■ 1 Hoheria angustifolia houhere, narrow-leaved lacebark (semi-decid) I ■ ½ ½ ■ ■ 1 Kunzea ericoides kanuka I ■ □ □ ■ ■ 1 Pittosporum eugenioides tarata, lemonwood F ■ ■ ½ ■ ½ 1 Plagianthus regius manatu, lowland ribbonwood (deciduous) F,I ■ ½ ½ ½ ■ 1 Podocarpus totara totara F ■ ½ ½ ■ ■ 2 Prumnopitys taxifolia matai, black pine F ■ ½ ■ ½ ■ 2 Pseudopanax crassifolius lancewood, horoeka F,N,B,I ■ ½ ½ ■ ■ 2 Sophora microphylla South Island kowhai F,I ■ ½ ½ ■ ■ t 2 SMALL TREES & TALL SHRUBS (> 3 m) Carpodetus serratus putaputaweta, marbleleaf F,I ½ ■ ■ ½ □ 2 Coprosma linariifolia linear-leaved coprosma, yellow-wood F ½ ■ ½ ½ ½ 2 Coprosma robusta karamu F ■ ■ ■ ½ ½ 1 Dodonaea viscosa akeake I ■ ½ □ ■ ■ 1-2* Griselinia littoralis kapuka, broadleaf F,I ■ ■ ½ ■ ■ 2 Leptospermum scoparium manuka, tea tree I ■ □ ■ ■ ■ 1 Lophomyrtus obcordata rohutu, NZ myrtle F,I ½ ■ ½ ½ ½ 2 Melicytus micranthus manakura, shrubby mahoe F,I ½ ■ ½ ½ □ 3 Melicytus ramiflorus mahoe, whiteywood -

Totara Cover Front
DISCLAIMER In producing this Bulletin reasonable care has been taken to ensure that all statements represent the best information available. However, the contents of this publication are not intended to be a substitute for specific specialist advice on any matter and should not be relied on for that purpose. NEW ZEALAND FOREST RESEARCH INSTITUTE LIMITED and its employees shall not be liable on any ground for any loss, damage, or liability incurred as a direct or indirect result of any reliance by any person upon information contained or opinions expressed in this work. To obtain further copies of this publication, or for information about Forest Research publications, please contact: Publications Officer Forest Research Private Bag 3020 Rotorua New Zealand telephone: +64 7 343 5899 facsimile: +64 7 343 5897 e-mail: [email protected] website: www.forestresearch.co.nz National Library of New Zealand Cataloguing-in-Publication data Bergin, D.O. (David O.) Totara establishment, growth, and management / David Bergin. (New Zealand Indigenous Tree Bulletin, 1176-2632; No.1) Includes bibliographic references. ISBN 0-478-11008-1 1. Podocarpus—New Zealand. 2. Forest management—New Zealand. I. New Zealand Forest Research Institute. II. Title. 585.3—dc 21 Production Team Jonathan Barran — photography Teresa McConchie — layout design John Smith — graphics Ruth Gadgil — technical editing Judy Griffith — editing and layout ISSN 1176-2632 ISBN 0-478-11008-1 © New Zealand Forest Research Institute Limited 2003 Front cover insert: Emergent totara and younger trees along the forest edge in Pureora Forest Park, with mixed shrub species edging the picnic area in the foreground.