Lessons from Nature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pollen-Based Temperature and Precipitation Records of the Past
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2017 Pollen-based temperature and precipitation records of the past 14,600 years in northern New Zealand (37°S) and their linkages with the Southern Hemisphere atmospheric circulation Ignacio Jara Victoria University of Wellington Rewi Newnham Victoria University of Wellington Brent V. Alloway University of Wollongong Janet M. Wilmshurst Landcare Research NZ Andrew Rees Victoria University of Wellington Publication Details Jara, I. A., Newnham, R. M., Alloway, B. V., Wilmshurst, J. M. & Rees, A. B.H. (2017). Pollen-based temperature and precipitation records of the past 14,600 years in northern New Zealand (37°S) and their linkages with the Southern Hemisphere atmospheric circulation. The oH locene: a major interdisciplinary journal focusing on recent environmental change, 27 (11), 1756-1768. Research Online is the open access institutional repository for the University of Wollongong. For further information contact the UOW Library: [email protected] Pollen-based temperature and precipitation records of the past 14,600 years in northern New Zealand (37°S) and their linkages with the Southern Hemisphere atmospheric circulation Abstract Regional vegetation, climate history, and local water table fluctuations for the past 14,600 years are reconstructed from pollen and charcoal records of an ombrogenous peatbog in northern New Zealand (38°S). A long-term warming trend between 14,600 and 10,000 cal. yr BP is punctuated by two brief plateaux between 14,200-13,800 and 13,500-12,000 cal. yr BP. Periods of relatively drier conditions are inferred between 14,000-13,400 and 12,000-10,000 cal. -
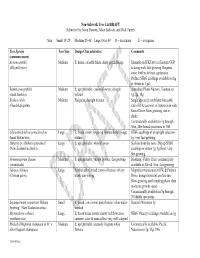
Recommended Non-Sidewalk Tree List DRAFT
Non-Sidewalk Tree List DRAFT Submitted by Jason Dewees, Mike Sullivan, and Dick Turner Size: Small 15-25’ Medium 25-40’ Large Over 40’ D = deciduous E = evergreen Tree Species Tree Size Design Characteristics Comments (common name) Acmena smithii Medium E; dense, colorful fruits; shiny green foliage Example on JFK Drive in Eastern GGP (lilly-pilly tree) is doing well; fast-growing. Requires some fertility for best appearance. Profuse SFBG seedlings available to dig or obtain in 1 gal. Banksia integrifolia Medium E; upright habit; creamy flowers; drought Australian Plants Nursery, Ventura, in (coast banksia) tolerant 1g, 5g, 15g Brahea edulis Medium Fan palm; drought tolerant Single specimen established on north (Guadalupe palm) side of JFK just west of intersection with Kezar Drive. Slow growing, sun or shade. Commercially available in 1g through 36in, 48in boxed specimens to 15ft Chiranthodendron pentadactylon Large E; broad crown; large red flowers, bold foliage SFBG seedlings of an upright selection: (hand flower tree) texture 1g; very fast-growing Hoheria sp. (Hoheria populnea? Large E; upright habit; white flowers Suckers from the roots. Dig up SFBG (New Zealand lacebark)) seedlings or obtain 1g, 5g there; very fast-growing Hymenosporum flavum Medium E; upright habit; yellow flowers; fast growing Boething, Valley Crest; commercially (sweetshade) available in 24in & 36in; fast-growing. Jubaea chilensis Large Feather palm; broad crown of leaves; silvery Magnificent specimen at JFK & Fuchsia (Chilean palm) trunk; sun-loving Drive; drought-tolerant, prefers sun. Slow-growing until trunking phase, then moderate growth speed. Commercially available in 5g through 20ft B&B specimens Leptospermum scoparium ‘Helene Small E; broad, low crown; pink flowers; often multi- Suncrest Nurseries 5g Strybing’ (New Zealand tea tree) trunked Metrosideros robusta Large E; broad dense crown; showy red flowers in SFBG Nursery seedlings available in 1g (northern rata) summer; slow & unusual but very well-adapted Michelia/Magnolia champaca or M. -

Species-Specific Basic Stem-Wood Densities for Twelve Indigenous Forest and Shrubland Species of Known Age, New Zealand
Marden et al. New Zealand Journal of Forestry Science (2021) 51:1 https://doi.org/10.33494/nzjfs512021x121x E-ISSN: 1179-5395 published on-line: 15/02/2021 Research Article Open Access New Zealand Journal of Forestry Science Species-specific basic stem-wood densities for twelve indigenous forest and shrubland species of known age, New Zealand Michael Marden1,*, Suzanne Lambie2 and Larry Burrows3 1 31 Haronga Road, Gisborne 4010, New Zealand 2 Manaaki Whenua – Landcare Research, Private Bag 3127, Hamilton 3240, New Zealand 3 Manaaki Whenua – Landcare Research, PO Box 69041, Lincoln 7640, New Zealand *Corresponding author: [email protected] (Received for publication 19 July 2019; accepted in revised form 26 January 2021) Abstract Background: Tree carbon estimates for New Zealand indigenous tree and shrub species are largely based on mean of sites throughout New Zealand. Yet stem-wood density values feed directly into New Zealand’s international and nationalbasic stem-wood greenhouse densities gas accounting. derived from We a limitedaugment number existing of publishedtrees, often basic of unspecified stem-wood age density and from data a limited with new number age- old, across 21 widely-distributed sites between latitudes 35° tospecific estimate values carbon for 12stocks. indigenous forest and shrubland species, including rarely obtained values for trees <6-years and 46° S, and explore relationships commonly used Methods: The volume of 478 whole stem-wood discs collected at breast height (BH) was determined by water displacement, oven dried, and weighed. Regression analyses were used to determine possible relationships between basic stem-wood density, and tree height, root collar diameter (RCD), and diameter at breast height (DBH). -

Recent Changes in the Names of New Zealand Tree and Shrub Species
-- -- - Recent changes in the names of New Zealand tree and shrub species - Since the publication of 'Flora of New Zealand' Volume 1 (A- iii) Podocarpus dacydioides Dacrycarpus ducydioides lan 1961),covering indigenous ferns, conifers and dicots, there (iii)Podocarpus ferrugzneus Prumnopitys ferruginea have been major advances in taxonomic research and the clas- Podocarpus spicatus Prumnopitys taxijolia sification of many plant groups revised accordingly. Most of (iv1 Dacrydium cupressinum (unchanged) these changes have been summarised in the Nomina Nova (v)Dacrydium bidwillii Halocarpus bidwillii series published in the New Zealand Journal of Botany (Edgar Dacrydium bijorme Halocarpus bijormis 1971, Edgar and Connor 1978, 1983) and are included in re- Dacrydium kirkii Halocarpus kirkii cent books on New Zealand plants ie.g. Eagle 1982, Wilson (vi)Dacydium colensoi Lagarostrobos colensoi 1982). A number of these name changes affect important (vii)Dacrydium intermediurn Lepidothamnus intermedius forest plants and as several of these new names are now start- Dacrydium laxijolium Lepidotbamnus laxijolius ing to appear in the scientific literature, a list of changes af- (viii)Phyllocladus trichomanoidi~(unchanged) fecting tree and shrub taxa are given here. As a large number Phyllocladus glaucus (unchanged) of the readers of New Zealand Forestry are likely to use Poole Phyllocladus alpinus Phyllocladus aspleniijolius and Adams' "Trees and Shrubs of New Zealand" as their var. alpinus* * main reference for New Zealand forest plants, all the name changes are related to the fourth impression of this book. * It has been suggested that the Colenso name P, cunnin- it is important to realise that not all botanists necessarily ghamii (1884)should take precedence over the later (18891 ark agree with one particular name and you are not obliged to use name (P. -

Plant Charts for Native to the West Booklet
26 Pohutukawa • Oi exposed coastal ecosystem KEY ♥ Nurse plant ■ Main component ✤ rare ✖ toxic to toddlers coastal sites For restoration, in this habitat: ••• plant liberally •• plant generally • plant sparingly Recommended planting sites Back Boggy Escarp- Sharp Steep Valley Broad Gentle Alluvial Dunes Area ment Ridge Slope Bottom Ridge Slope Flat/Tce Medium trees Beilschmiedia tarairi taraire ✤ ■ •• Corynocarpus laevigatus karaka ✖■ •••• Kunzea ericoides kanuka ♥■ •• ••• ••• ••• ••• ••• ••• Metrosideros excelsa pohutukawa ♥■ ••••• • •• •• Small trees, large shrubs Coprosma lucida shining karamu ♥ ■ •• ••• ••• •• •• Coprosma macrocarpa coastal karamu ♥ ■ •• •• •• •••• Coprosma robusta karamu ♥ ■ •••••• Cordyline australis ti kouka, cabbage tree ♥ ■ • •• •• • •• •••• Dodonaea viscosa akeake ■ •••• Entelea arborescens whau ♥ ■ ••••• Geniostoma rupestre hangehange ♥■ •• • •• •• •• •• •• Leptospermum scoparium manuka ♥■ •• •• • ••• ••• ••• ••• ••• ••• Leucopogon fasciculatus mingimingi • •• ••• ••• • •• •• • Macropiper excelsum kawakawa ♥■ •••• •••• ••• Melicope ternata wharangi ■ •••••• Melicytus ramiflorus mahoe • ••• •• • •• ••• Myoporum laetum ngaio ✖ ■ •••••• Olearia furfuracea akepiro • ••• ••• •• •• Pittosporum crassifolium karo ■ •• •••• ••• Pittosporum ellipticum •• •• Pseudopanax lessonii houpara ■ ecosystem one •••••• Rhopalostylis sapida nikau ■ • •• • •• Sophora fulvida west coast kowhai ✖■ •• •• Shrubs and flax-like plants Coprosma crassifolia stiff-stemmed coprosma ♥■ •• ••••• Coprosma repens taupata ♥ ■ •• •••• •• -

Identification De Polyphénols, Évaluation De Leur Activité Antioxydante Et Étude De Leurs Propriétés Biologiques François Muanda Nsemi
Identification de polyphénols, évaluation de leur activité antioxydante et étude de leurs propriétés biologiques François Muanda Nsemi To cite this version: François Muanda Nsemi. Identification de polyphénols, évaluation de leur activité antioxydante et étude de leurs propriétés biologiques. Biologie végétale. Université Paul Verlaine - Metz, 2010. Français. NNT : 2010METZ011S. tel-01752680 HAL Id: tel-01752680 https://hal.univ-lorraine.fr/tel-01752680 Submitted on 29 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. AVERTISSEMENT Ce document est le fruit d'un long travail approuvé par le jury de soutenance et mis à disposition de l'ensemble de la communauté universitaire élargie. Il est soumis à la propriété intellectuelle de l'auteur. Ceci implique une obligation de citation et de référencement lors de l’utilisation de ce document. D'autre part, toute contrefaçon, plagiat, reproduction illicite encourt une poursuite pénale. Contact : [email protected] LIENS Code de la Propriété Intellectuelle. articles L 122. 4 Code de -

Patterns of Flammability Across the Vascular Plant Phylogeny, with Special Emphasis on the Genus Dracophyllum
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy at Lincoln University by Xinglei Cui Lincoln University 2020 Abstract of a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of philosophy. Abstract Patterns of flammability across the vascular plant phylogeny, with special emphasis on the genus Dracophyllum by Xinglei Cui Fire has been part of the environment for the entire history of terrestrial plants and is a common disturbance agent in many ecosystems across the world. Fire has a significant role in influencing the structure, pattern and function of many ecosystems. Plant flammability, which is the ability of a plant to burn and sustain a flame, is an important driver of fire in terrestrial ecosystems and thus has a fundamental role in ecosystem dynamics and species evolution. However, the factors that have influenced the evolution of flammability remain unclear. -

Agathis Robusta and Agathis Australis Friends Friends
Plants in Focus, December 2016 Agathis robusta and Agathis australis Friends of GeelongBotanic Left: The Qld Kauri Agathis robusta, planted in the Albury BG in 1910, is the largest recorded in the Big Tree Register. Note gardener. [1] Right: The NZ Kauri Agathis australis, named Tane Mahuta (Lord of the Forest), in the Waipoua Forest is the largest known in NZ. Photo: Prof. Chen Hualin, CC BY-SA 4.0, zh.wikipedia.org Kauris (Agathis sp.) are conifers Conifers, along with the other Gymnosperms (Cycads and Ginkgoes), first appeared about 300 Ma (Million years ago) at the end of the Carboniferous when the world’s coal deposits were being laid down with the remains of the spore-producing trees of that period. The early conifers looked like modern Araucaria. These trees spread throughout the world and displaced their predecessors. The age of the seed plants had arrived. The conifers are a hardy lot. They survived the largest mass extinction the earth has known, 252 Ma, at the end of the Permian Period. But more challenges lay ahead. Sometime in the next 50 Myr (Million years) one of Gymnosperms gave rise to the flowering plants, the Angiosperms. By 100 Ma, in the Cretaceous period, Angiosperms were widespread. And so the battle began - and still continues to this day. The flowering plants have many features that make them more successful in many environments, so their take-over of many habitats was complete by about 65 Ma at the end of the age of the dinosaurs. But in the world’s harsh environments the conifers continue to not just survive, but flourish. -

Pollination Drop in Relation to Cone Morphology in Podocarpaceae: a Novel Reproductive Mechanism Author(S): P
Pollination Drop in Relation to Cone Morphology in Podocarpaceae: A Novel Reproductive Mechanism Author(s): P. B. Tomlinson, J. E. Braggins, J. A. Rattenbury Source: American Journal of Botany, Vol. 78, No. 9 (Sep., 1991), pp. 1289-1303 Published by: Botanical Society of America Stable URL: http://www.jstor.org/stable/2444932 . Accessed: 23/08/2011 15:47 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Botanical Society of America is collaborating with JSTOR to digitize, preserve and extend access to American Journal of Botany. http://www.jstor.org AmericanJournal of Botany 78(9): 1289-1303. 1991. POLLINATION DROP IN RELATION TO CONE MORPHOLOGY IN PODOCARPACEAE: A NOVEL REPRODUCTIVE MECHANISM' P. B. TOMLINSON,2'4 J. E. BRAGGINS,3 AND J. A. RATTENBURY3 2HarvardForest, Petersham, Massachusetts 01366; and 3Departmentof Botany, University of Auckland, Auckland, New Zealand Observationof ovulatecones at thetime of pollinationin the southernconiferous family Podocarpaceaedemonstrates a distinctivemethod of pollencapture, involving an extended pollinationdrop. Ovules in all generaof the family are orthotropousand singlewithin the axil of each fertilebract. In Microstrobusand Phyllocladusovules are-erect (i.e., the micropyle directedaway from the cone axis) and are notassociated with an ovule-supportingstructure (epimatium).Pollen in thesetwo genera must land directly on thepollination drop in theway usualfor gymnosperms, as observed in Phyllocladus.In all othergenera, the ovule is inverted (i.e., the micropyleis directedtoward the cone axis) and supportedby a specializedovule- supportingstructure (epimatium). -

La Citeset Le Bois
La CITES et le Bois Ce guide couvre les principales espèces de bois réglementées par la Convention sur le commerce international des espèces de faune and de flore La CITES et le Bois sauvage menacées d’extinction (CITES). Il fournit des informations sur des questions clés relatives à la mise Guide d’espèces d’arbres inscrites aux Annexes CITES en application de la Convention pour ce groupe important de plantes. Rédigé pour des non-experts, il inclut des sections individuelles couvrant les espèces trouvées en quantités importantes dans le Madeleine Groves Madeleine Groves commerce, avec les details de leurs répartitions, utilisations, parties et produits dans le commerce, et leurs noms scientifiques et communs. et Des sections supplémentaires couvrant Catherine Rutherford l’identification et le mesurage du bois, des orientations en matière de documentation CITES, et des ressources clés. shop.kew.org/kewbooksonline ISBN 978-1-84246-637-7 Madeleine Groves Catherine Rutherford 9 781842 466377 La CITES et le Bois Guide d’espèces d’arbres inscrites aux Annexes CITES Madeleine Groves Catherine Rutherford © Office fédéral de la sécurité alimentaire et des affaires vétérinaires (OSAV), Confédération suisse. Illustrations et photographies © Royal Botanic Gardens, Kew sauf si autrement mentionné dans les légendes. Les auteurs ont fait valoir leur droit à être identifiés comme étant les auteurs de ces travaux conformément à la loi du Royaume-Uni de 1988 en matière de « Copyright, Design & Patents ». Tous droits réservés. Aucune partie de la présente publication ne peut être reproduite, stockée dans un système de récupération, ou transmise, quelle que soit la forme, ou par un quelconque moyen électronique, mécanique, photocopie, enregistrement ou autre, sans le consentement écrit de l’éditeur, sauf en conformité avec les dispositions de la loi du Royaume-Uni de 1988 en matière de « Copyright, Design & Patents ». -

A Quantitative Assessment of Shoot Flammability for 60 Tree and Shrub Species Supports Rankings Based on Expert Opinion
CSIRO PUBLISHING International Journal of Wildland Fire 2016, 25, 466–477 http://dx.doi.org/10.1071/WF15047 A quantitative assessment of shoot flammability for 60 tree and shrub species supports rankings based on expert opinion Sarah V. WyseA,B,G, George L. W. PerryA,C, Dean M. O’ConnellD, Phillip S. HollandD, Monique J. WrightD, Catherine L. HostedD,E, Samuel L. WhitelockD, Ian J. GearyD,F, Ke´vinJ.L.MaurinD and Timothy J. CurranD ASchool of Environment, University of Auckland, Private Bag 92019 Auckland 1142, New Zealand. BRoyal Botanic Gardens Kew, Wakehurst Place, RH17 6TN, UK. CSchool of Biological Sciences, University of Auckland, Private Bag 92019 Auckland 1142, New Zealand. DEcology Department, Lincoln University, PO Box 85084, Lincoln 7647, Canterbury, New Zealand. EWai-Ora Forest Landscapes Ltd, 48 Watsons Road, Harewood 8051, Christchurch, New Zealand. FDepartment of Geology, University of Otago, PO Box 56 Dunedin 9054, New Zealand. GCorresponding author. Email: [email protected] Abstract. Fire is an important ecological disturbance in vegetated ecosystems across the globe, and also has considerable impacts on human infrastructure. Vegetation flammability is a key bottom-up control on fire regimes and on the nature of individual fires. Although New Zealand (NZ) historically had low fire frequencies, anthropogenic fires have considerably impacted indigenous vegetation as humans used fire extensively to clear forests. Few studies of vegetation flammability have been undertaken in NZ and only one has compared the flammability of indigenous plants; this was a qualitative assessment derived from expert opinion. We addressed this knowledge gap by measuring the flammability of terminal shoots from a range of trees and shrubs found in NZ. -

Pollination in New Zealand
2.11 POLLINATION IN NEW ZEALAND POLLINATION IN NEW ZEALAND Linda E. Newstrom-Lloyd Landcare Research, PO Box 69040, Lincoln 7640, New Zealand ABSTRACT: Pollination by animals is a crucial ecosystem service. It underpins New Zealand’s agriculture-dependent economy yet has hitherto received little attention from a commercial perspective except where pollination clearly limits crop yield. In part this has been because background pollination by feral honey bees (Apis mellifera) and other unmanaged non-Apis pollinators has been adequate. However, as pollinators decline throughout the world, the consequences for food production and national economies have led to increasing research on how to prevent further declines and restore pollination services. In New Zealand, managed honey bees are the most important pollinators of most commercial crops including pasture legumes, but introduced bumble bees can be more important in some crops and are increasingly being used as managed colonies. In addition, New Zealand has several other introduced bees and a range of solitary native bees, some of which offer prospects for development as managed colonies. Diverse other insects and some vertebrates also contribute to background pollination in both natural and agricultural ecosystems. However, New Zealand’s depend- ence on managed honey bees makes it vulnerable to four major threats facing these bees: diseases, pesticides, a limited genetic base for breeding varroa-resistant bees, and declining fl oral resources. To address the fourth threat, a preliminary list of bee forage plants has been developed and published online. This lists species suitable for planting to provide abundant nectar and high-quality pollen during critical seasons.