Crocanthemum Canadense (L.) Britt
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

2015 Summary of Changes to Endangered, Threatened, And
2015 Update to State Listed Species The Department of Energy and Environmental Protection (DEEP) is required to review, at least every five years, the designation of species as endangered, threatened, or of special concern to determine whether species should be: (1) added or removed from the list; or, if necessary, (2) change the designation from one category to another. The following is a summary of the changes to the State Endangered Species list (DEEP Regulations Sections 26‐306‐4, 26‐306‐5, and 26‐306‐6) that became effective on August 5, 2015. The complete list can be found on the DEEP website. Summary of Amphibian Changes New species added Necturus maculosus, Mudpuppy added as Special Concern Summary of Reptile Changes New species added Clemmys guttata, Spotted turtle added as Special Concern Malaclemys terrapin terrapin, Northern diamondback terrapin added as Special Concern Taxonomic Changes Eumeces fasciatus, Five‐lined skink changed to Plestiodon fasciatus Liochlorophis vernalis, Smooth green snake changed to Opheodrys vernalis Summary of Bird Changes Northern diamondback terrapin Status Changes Falco sparverius, American kestrel downlisted to Special Concern Progne subis, Purple martin downlisted to Special Concern Sturnella magna, Eastern meadowlark uplisted to Threatened New species added Accipiter gentilis, Northern goshawk added as Threatened Setophaga cerulea, Cerulean warbler added as Special Concern Species delisted Anas discors, Blue‐winged teal Laterallus jamaicensis, Black rail Cerulean warbler Taxonomic changes Parula americana, Northern parula changed to Setophaga americana 1 Summary of Mammal Changes Status Changes Myotis leibii, Eastern small‐footed bat uplisted to Endangered New Species Added Myotis lucifugus, Little brown bat added as Endangered Myotis septentrionalis, Northern long‐eared bat added as Endangered (also Federally Threatened) Perimyotis subflavus, Tri‐colored bat added as Endangered Taxonomic Changes Phocoena phocoena, Harbor porpoise changed to Phocoena Northern long‐eared bat phocoena ssp. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

The British Forms of Tuberaria Guttata (L.) Fourreau
THE BRITISH FORMS OF TUBERARIA GUTTATA (L.) FOURREAU By M. C. F. PROCTOR Department of Botany, University of Exeter ABSTRACT An account is given of the variation in British Tuberaria guttata, and of its previous taxonomic treat ment: the taxonomic value of various characters is examined. Most of the Welsh and Irish plants includ ing the type population of Helianthemum breweri Planch. differ from T. guttata as it occurs in the Channel Isles and northern France in their shorter stature, the more common presence of bracts and other characters. All the characters said to distinguish breweri intergrade continuously with those of typical guttata in both herbarium and cultivated material, and are only loosely correlated. The more compact Welsh and Irish plants appear to be comparable with plants in similar exposed coastal habitats in north-west France. It is concluded that T. guttata shows ecotypic differentiation in relation to exposure on the Atlantic coast of Europe, and that the populations combining short diffuse habit and numerous bracts may be of polytopic origin. It is suggested that they should not be given formal taxonomic recognition. 1. INTRODUCTION Like a number of other widespread Mediterranean species, Tuberaria guttata extends northwards up the west coast of Europe to a northern limit in the British Isles. Up to the north coast of France its distribution is more or less continuous, and the Channel Islands lie on the northern fringe of this essentially continuous area. But north of the English Channel its range is disjunct, and it occurs only in widely separated colonies on the coasts of north Wales and western Ireland. -

Endemic Plant Species Tyler M
Exclusion of introduced deer increases size and seed production success in an island-endemic plant species Tyler M. Dvorak & Amy E. Catalano Conservation Department, Catalina Island Conservancy, P.O. Box 2739, Avalon, California 90704 Keywords Abstract Crocanthemum greenei, deer, exclosure, herbivory, invasion, island. The presence of extra-local invaders, such as the southern California mule deer (Odocoileus hemionus) on Santa Catalina Island, may contribute to more selec- Correspondence tive and insidious effects within the unique ecosystems that have evolved in Tyler M. Dvorak, Catalina Island Conservancy, their absence. Studies at the species level may detect effects not noticed in P.O. Box 2739, Avalon, CA 90704 broader, community level vegetation monitoring or help tease apart differences Tel: +1 310 510 1299 x233; in the level of effect among the various ecological components of an invaded Fax: +1 310 510 1729; system. In this initial study, we measured the impacts of herbivory by mule E-mail: [email protected] deer, a species native to analogous habitats on the adjacent mainland, on size Funding Information and seed production success for Crocanthemum greenei (island rush-rose), a We recognize the United States Fish & federally listed sub-shrub that is not present on mainland California. We found Wildlife Service (#F10AC00790), Seaver deer exclusion resulted in an overall increase in stem measurement of 18.8 cm. Institute, and Marisla Foundation for their Exclosure populations exhibited complete seed production success, whereas crucial support. control populations showed significantly reduced success and exhibited com- plete failure within 58% of populations. These results show that the introduced Received: 8 January 2015; Revised: 6 November 2015; Accepted: 25 November mule deer on Santa Catalina Island are negatively affecting a federally threat- 2015 ened plant species. -

Ectomycorrhizal Fungal Communities at Forest Edges 93, 244–255 IAN A
Journal of Blackwell Publishing, Ltd. Ecology 2005 Ectomycorrhizal fungal communities at forest edges 93, 244–255 IAN A. DICKIE and PETER B. REICH Department of Forest Resources, University of Minnesota, St Paul, MN, USA Summary 1 Ectomycorrhizal fungi are spatially associated with established ectomycorrhizal vegetation, but the influence of distance from established vegetation on the presence, abundance, diversity and community composition of fungi is not well understood. 2 We examined mycorrhizal communities in two abandoned agricultural fields in Minnesota, USA, using Quercus macrocarpa seedlings as an in situ bioassay for ecto- mycorrhizal fungi from 0 to 20 m distance from the forest edge. 3 There were marked effects of distance on all aspects of fungal communities. The abundance of mycorrhiza was uniformly high near trees, declined rapidly around 15 m from the base of trees and was uniformly low at 20 m. All seedlings between 0 and 8 m distance from forest edges were ectomycorrhizal, but many seedlings at 16–20 m were uninfected in one of the two years of the study. Species richness of fungi also declined with distance from trees. 4 Different species of fungi were found at different distances from the edge. ‘Rare’ species (found only once or twice) dominated the community at 0 m, Russula spp. were dominants from 4 to 12 m, and Astraeus sp. and a Pezizalean fungus were abundant at 12 m to 20 m. Cenococcum geophilum, the most dominant species found, was abundant both near trees and distant from trees, with lowest relative abundance at intermediate distances. 5 Our data suggest that seedlings germinating at some distance from established ecto- mycorrhizal vegetation (15.5 m in the present study) have low levels of infection, at least in the first year of growth. -

Tuberaria Guttata (L.) Fourr
Tuberaria guttata (L.) Fourr Spotted Rock-rose Tuberaria guttata flowers from May to June and is best searched for on warm and sunny mornings when the bright yellow, purple- blotched petals open widely and are readily visible. Populations are known from northern Wales, western and south-western Ireland, the Channel Islands and western Scotland, although there are doubts about its provenance at the sole Scottish locality. It occurs at coastal locations in exposed, well-drained rocky outcrops on moderately acidic shallow peats, typically where there is sparse vegetation cover, making populations particularly vulnerable to the encroachment of more competitive vegetation. It is assessed as Near Threatened in Britain, but is of Least Concern in Wales. ©Joh n Crellin IDENTIFICATION HABITATS In flower T. guttata is unmistakable, with bright yellow petals In Britain and Ireland T. guttata grows on exposed rocky that have purple blotches and opposite leaves that are three- outcrops in bare open stony or peaty patches amongst species- v eined and turn reddish with age. Plants are variable in size, poor Calluna vulgaris-Scilla verna and C. vulgaris-Erica ranging from solitary flowers on short stems (1-2 cm) in cinerea heathland (NVC H7, H10), and more rarely Festuca exposed sites to much taller (to 20 cm) branched stems with ovina-Agrostis capillaris-Galium saxatile (NVC U4d) multiple flowers in more sheltered localities. grassland. Tuberaria guttata tends to be concentrated where there is a SIMILAR SPECIES sparse cov er of grasses, ericaceous shrubs and other small herbs, and often grows in a thin carpet of mosses and lichens. -

Greek Island Odyssey Holiday Report 2013
Greek Island Odyssey Holiday Report 2013 Day 1: Saturday 20th April As our plane came in to land at Rhodes airport the wildlife spotting began! We had a good view of a female Marsh Harrier and Little Egret over the nearby river. Then, on the drive to the hotel, we saw a Wood Sandpiper on the same river by the road bridge. Upon our arrival in the medieval old town Andy and Denise made a quick foray into the moat and town and found Starred Agamas, Oertzen’s Rock Lizards, a Dahl’s Whip Snake and Large Wall Brown butterflies. It was late evening by then and so we sat at a local taverna for our first traditional Greek mezedes meal and discussed plans for the week ahead over a civilized glass of wine. Day 2: Sunday 21st April After a hearty breakfast at the hotel we set off on our first Anatolian Worm Lizard full day of exploration. Our first stop was the archaeological park at Monte Smith. After parking the car and with lots of butterflies flying around us, it was hard to know just what to look at first. Andy diverted our attention, announcing that he had found an Anatolian Worm Lizard, a strange creature looking more like a worm than a lizard and which is found in Turkey and Greece. On Rhodes it is recorded only in the northern parts of the island. Lesser Fiery Copper We then moved on to watch the butterflies. The first two we identified were male and female Lesser Fiery Coppers, soon followed by Eastern Bath White, and Clouded yellow. -

THE IRISH RED DATA BOOK 1 Vascular Plants
THE IRISH RED DATA BOOK 1 Vascular Plants T.G.F.Curtis & H.N. McGough Wildlife Service Ireland DUBLIN PUBLISHED BY THE STATIONERY OFFICE 1988 ISBN 0 7076 0032 4 This version of the Red Data Book was scanned from the original book. The original book is A5-format, with 168 pages. Some changes have been made as follows: NOMENCLATURE has been updated, with the name used in the 1988 edition in brackets. Irish Names and family names have also been added. STATUS: There have been three Flora Protection Orders (1980, 1987, 1999) to date. If a species is currently protected (i.e. 1999) this is stated as PROTECTED, if it was previously protected, the year(s) of the relevant orders are given. IUCN categories have been updated as follows: EN to CR, V to EN, R to V. The original (1988) rating is given in brackets thus: “CR (EN)”. This takes account of the fact that a rare plant is not necessarily threatened. The European IUCN rating was given in the original book, here it is changed to the UK IUCN category as given in the 2005 Red Data Book listing. MAPS and APPENDIX have not been reproduced here. ACKNOWLEDGEMENTS We are most grateful to the following for their help in the preparation of the Irish Red Data Book:- Christine Leon, CMC, Kew for writing the Preface to this Red Data Book and for helpful discussions on the European aspects of rare plant conservation; Edwin Wymer, who designed the cover and who, as part of his contract duties in the Wildlife Service, organised the computer applications to the data in an efficient and thorough manner. -

Malvales Nymphaeales Austrobaileyales
Amborellales Malvales Nymphaeales Austrobaileyales Acorales G Eenzaadlobbigen G Alismatales Petrosaviales Huerteales Pandanales Een recente ontwikkeling is het Dioscoreales Dipentodontaceae in een nieuw Liliales Asparagales hout- en anatomische kenmerke 2 geslachten en 5 soorten van b Arecales en samengestelde bladeren, die G Commeliniden G Dasypogonales Poales werden geplaatst. De Dipentod Commelinales sinicus, een boom uit China en Zingiberales die vroeger in de Violales werd Ceratophyllales Malvales Chloranthales De Malvales zijn voor het meren Canellales warme streken. Ze hebben vers Piperales G Magnoliiden G De bast is nogal eens vezelig, st Magnoliales veel voor. De kroonbladen ligge Laurales Ze hebben meestal een lange st Ranunculales De zaden en de binnenkant van Sabiales bezet. Deze orde omvatte al de Proteales Trochodendrales Dipterocarpaceae, Bixaceae, Ne Buxales Sphaerosepalaceae. De Lindefam Gunnerales Bombacaceae zijn nu opgenom Berberidopsidales (Malvaceae). De Muntingiaceae Dilleniales afgesplitst. Nieuwkomers in de Caryophyllales Santalales (Cistaceae), uit de Violales, en d Saxifragales (Thymelaeaceae) uit de Euphorb Cytinaceae (vroeger Rafflesiales G Geavanceerde tweezaadlobbigen G Vitales Crossosomatales ook in deze orde thuis. Geraniales Myrtales Sapindales Zygophyllales De meeste soorten in deze orde Celastrales houtige gewassen, vaak met sam Malpighiales G Fabiden G Oxalidales Fabales Rosales Bixaceae G Rosiden G Cucurbitales Malvaceae Fagales Muntingiaceae Cistaceae Huerteales Dipterocarpaceae G G Malviden Brassicales -

Vascular Plants of Santa Cruz County, California
ANNOTATED CHECKLIST of the VASCULAR PLANTS of SANTA CRUZ COUNTY, CALIFORNIA SECOND EDITION Dylan Neubauer Artwork by Tim Hyland & Maps by Ben Pease CALIFORNIA NATIVE PLANT SOCIETY, SANTA CRUZ COUNTY CHAPTER Copyright © 2013 by Dylan Neubauer All rights reserved. No part of this publication may be reproduced without written permission from the author. Design & Production by Dylan Neubauer Artwork by Tim Hyland Maps by Ben Pease, Pease Press Cartography (peasepress.com) Cover photos (Eschscholzia californica & Big Willow Gulch, Swanton) by Dylan Neubauer California Native Plant Society Santa Cruz County Chapter P.O. Box 1622 Santa Cruz, CA 95061 To order, please go to www.cruzcps.org For other correspondence, write to Dylan Neubauer [email protected] ISBN: 978-0-615-85493-9 Printed on recycled paper by Community Printers, Santa Cruz, CA For Tim Forsell, who appreciates the tiny ones ... Nobody sees a flower, really— it is so small— we haven’t time, and to see takes time, like to have a friend takes time. —GEORGIA O’KEEFFE CONTENTS ~ u Acknowledgments / 1 u Santa Cruz County Map / 2–3 u Introduction / 4 u Checklist Conventions / 8 u Floristic Regions Map / 12 u Checklist Format, Checklist Symbols, & Region Codes / 13 u Checklist Lycophytes / 14 Ferns / 14 Gymnosperms / 15 Nymphaeales / 16 Magnoliids / 16 Ceratophyllales / 16 Eudicots / 16 Monocots / 61 u Appendices 1. Listed Taxa / 76 2. Endemic Taxa / 78 3. Taxa Extirpated in County / 79 4. Taxa Not Currently Recognized / 80 5. Undescribed Taxa / 82 6. Most Invasive Non-native Taxa / 83 7. Rejected Taxa / 84 8. Notes / 86 u References / 152 u Index to Families & Genera / 154 u Floristic Regions Map with USGS Quad Overlay / 166 “True science teaches, above all, to doubt and be ignorant.” —MIGUEL DE UNAMUNO 1 ~ACKNOWLEDGMENTS ~ ANY THANKS TO THE GENEROUS DONORS without whom this publication would not M have been possible—and to the numerous individuals, organizations, insti- tutions, and agencies that so willingly gave of their time and expertise. -
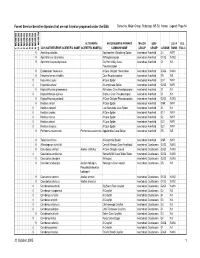
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR