Neuromodulation of the Cerebellum Rescues Movement in a Mouse Model of Ataxia
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
J Neurosurg 115:971–984, 2011 Magnetic resonance imaging techniques for visualization of the subthalamic nucleus A review ELLEN J. L. BRUNENbeRG, M.SC.,1 BRAM PLATEL, PH.D.,2 PAUL A. M. HOFMAN, PH.D., M.D.,3 BART M. TER HAAR ROmeNY, PH.D.,1,4 AND VeeRLE VIsseR-VANdeWALLE, PH.D., M.D.5,6 1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven; Departments of 3Radiology, 4Biomedical Engineering, and 5Neurosurgery, and 6Maastricht Institute for Neuromodulative Development, Maastricht University Medical Center, Maastricht, The Netherlands; and 2Fraunhofer MEVIS, Bremen, Germany The authors reviewed 70 publications on MR imaging–based targeting techniques for identifying the subtha- lamic nucleus (STN) for deep brain stimulation in patients with Parkinson disease. Of these 70 publications, 33 presented quantitatively validated results. There is still no consensus on which targeting technique to use for surgery planning; methods vary greatly between centers. Some groups apply indirect methods involving anatomical landmarks, or atlases incorporating ana- tomical or functional data. Others perform direct visualization on MR imaging, using T2-weighted spin echo or inver- sion recovery protocols. The combined studies do not offer a straightforward conclusion on the best targeting protocol. Indirect methods are not patient specific, leading to varying results between cases. On the other hand, direct targeting on MR imaging suffers from lack of contrast within the subthalamic region, resulting in a poor delineation of the STN. These defi- ciencies result in a need for intraoperative adaptation of the original target based on test stimulation with or without microelectrode recording. It is expected that future advances in MR imaging technology will lead to improvements in direct targeting. -

Bilateral Cerebellar Dysfunctions in a Unilateral Meso-Diencephalic Lesion
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.44.4.361 on 1 April 1981. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1981, 44, 361-363 Short report Bilateral cerebellar dysfunctions in a unilateral meso-diencephalic lesion D VON CRAMON From the Max-Planck-Institute for Psychiatry, Munich, Germany SUMMARY The clinical syndrome of a 65-year-old patient with a slit-shaped right-sided meso- diencephalic lesion was analysed. A cerebellar syndrome with limb-kinetic ataxia, intention tremor and hypotonicity in all extremities as well as ataxic dysarthria was found. The disruption of the two cerebello-(rubro)-thalamic pathways probably explained the signs of bilateral cere- bellar dysfunction. The uncrossed ascending limb of the right, and the crossed one of the left brachium conjunctivum may have been damaged by the unilateral lesion extending between caudal midbrain and dorsal thalamus. Protected by copyright. Most of the fibres which constitute the superior general hospital where neurological examination cerebellar peduncle leave the cerebellum and showed bilateral miosis, convergent strabism, vertical originate in cells of the dentate nucleus but also gaze paresis on upward gaze with gaze-paretic nystag- arise from neurons of the globose and emboli- mus, flaccid sensori-motor hemiparesis with increased stretch reflexes and Babinski sign on the left side, forme nuclei. The crossed ascending fibres of the and dysmetric movements of the right upper extremity. brachia conjunctiva constitute the major outflow The CT scan showed an acute haemorrhage in the from the cerebellum, they form the cerebello- right mesodiencephalic area. On 19 February 1979 (rubro)-thalamic and dentato-thalamic tracts.' the patient was admitted to our department. -

Differentiation of the Cerebellum 2463
Development 128, 2461-2469 (2001) 2461 Printed in Great Britain © The Company of Biologists Limited 2001 DEV1660 Inductive signal and tissue responsiveness defining the tectum and the cerebellum Tatsuya Sato, Isato Araki‡ and Harukazu Nakamura* Department of Molecular Neurobiology, Institute of Development, Aging and Cancer, Seiryo-machi 4-1, Aoba-ku, Sendai 980- 8575, Japan ‡Present address: Department of Neurobiology, University of Heidelberg, Im Neuenheimer Feld 364, D-69120 Heidelberg, Germany *Author for correspondence (e-mail: [email protected]) Accepted 11 April 2001 SUMMARY The mes/metencephalic boundary (isthmus) has an Fgf8b repressed Otx2 expression, but upregulated Gbx2 and organizing activity for mesencephalon and metencephalon. Irx2 expression in the mesencephalon. As a result, Fgf8b The candidate signaling molecule is Fgf8 whose mRNA is completely changed the fate of the mesencephalic alar plate localized in the region where the cerebellum differentiates. to cerebellum. Quantitative analysis showed that Fgf8b Responding to this signal, the cerebellum differentiates in signal is 100 times stronger than Fgf8a signal. Co- the metencephalon and the tectum differentiates in the transfection of Fgf8b with Otx2 indicates that Otx2 is a key mesencephalon. Based on the assumption that strong Fgf8 molecule in mesencephalic generation. We have shown by signal induces the cerebellum and that the Fgf8b signal is RT-PCR that both Fgf8a and Fgf8b are expressed, Fgf8b stronger than that of Fgf8a, we carried out experiments to expression prevailing in the isthmic region. The results all misexpress Fgf8b and Fgf8a in chick embryos. Fgf8a did not support our working hypothesis that the strong Fgf8 signal affect the expression pattern of Otx2, Gbx2 or Irx2. -

Occurrence of Long-Term Depression in the Cerebellar Flocculus During Adaptation of Optokinetic Response Takuma Inoshita, Tomoo Hirano*
SHORT REPORT Occurrence of long-term depression in the cerebellar flocculus during adaptation of optokinetic response Takuma Inoshita, Tomoo Hirano* Department of Biophysics, Graduate School of Science, Kyoto University, Sakyo-ku, Japan Abstract Long-term depression (LTD) at parallel fiber (PF) to Purkinje cell (PC) synapses has been considered as a main cellular mechanism for motor learning. However, the necessity of LTD for motor learning was challenged by demonstration of normal motor learning in the LTD-defective animals. Here, we addressed possible involvement of LTD in motor learning by examining whether LTD occurs during motor learning in the wild-type mice. As a model of motor learning, adaptation of optokinetic response (OKR) was used. OKR is a type of reflex eye movement to suppress blur of visual image during animal motion. OKR shows adaptive change during continuous optokinetic stimulation, which is regulated by the cerebellar flocculus. After OKR adaptation, amplitudes of quantal excitatory postsynaptic currents at PF-PC synapses were decreased, and induction of LTD was suppressed in the flocculus. These results suggest that LTD occurs at PF-PC synapses during OKR adaptation. DOI: https://doi.org/10.7554/eLife.36209.001 Introduction The cerebellum plays a critical role in motor learning, and a type of synaptic plasticity long-term *For correspondence: depression (LTD) at parallel fiber (PF) to Purkinje cell (PC) synapses has been considered as a primary [email protected]. cellular mechanism for motor learning (Ito, 1989; Hirano, 2013). However, the hypothesis that LTD ac.jp is indispensable for motor learning was challenged by demonstration of normal motor learning in Competing interests: The rats in which LTD was suppressed pharmacologically or in the LTD-deficient transgenic mice authors declare that no (Welsh et al., 2005; Schonewille et al., 2011). -

Neocortex: Consciousness Cerebellum
Grey matter (chips) White matter (the wiring: the brain mainly talks to itself) Neocortex: consciousness Cerebellum: unconscious control of posture & movement brains 1. Golgi-stained section of cerebral cortex 2. One of Ramon y Cajal’s faithful drawings showing nerve cell diversity in the brain cajal Neuropil: perhaps 1 km2 of plasma membrane - a molecular reaction substrate for 1024 voltage- and ligand-gated ion channels. light to Glia: 3 further cell types 1. Astrocytes: trophic interface with blood, maintain blood brain barrier, buffer excitotoxic neurotransmitters, support synapses astros Oligodendrocytes: myelin insulation oligos Production persists into adulthood: radiation myelopathy 3. Microglia: resident macrophages of the CNS. Similarities and differences with Langerhans cells, the professional antigen-presenting cells of the skin. 3% of all cells, normally renewed very slowly by division and immigration. Normal Neurosyphilis microglia Most adult neurons are already produced by birth Peak synaptic density by 3 months EMBRYONIC POSTNATAL week: 0 6 12 18 24 30 36 Month: 0 6 12 18 24 30 36 Year: 4 8 12 16 20 24 Cell birth Migration 2* Neurite outgrowth Synaptogenesis Myelination 1* Synapse elimination Modified from various sources inc: Andersen SL Neurosci & Biobehav Rev 2003 Rakic P Nat Rev Neurosci 2002 Bourgeois Acta Pediatr Suppl 422 1997 timeline 1 Synaptogenesis 100% * Rat RTH D BI E A Density of synapses in T PUBERTY primary visual cortex H at different times post- 0% conception. 100% (logarithmic scale) RTH Cat BI D E A T PUBERTY H The density values equivalent 0% to 100% vary between species 100% but in Man the peak value is Macaque 6 3 RTH 350 x10 synapses per mm BI D E PUBERTY A T The peak rate of synapse H formation is at birth in the 0% macaque: extrapolating to 100% the entire cortex, this Man RTH BI amounts to around 800,000 D E synapses formed per sec. -

Cerebellar Granule Cells in Culture
Proc. Nati. Acad. Sci. USA Vol. 83, pp. 4957-4961, July 1986 Neurobiology Cerebellar granule cells in culture: Monosynaptic connections with Purkinje cells and ionic currents (excitatory postsynaptic potential/patch-clamp) ToMoo HIRANO, YOSHIHIRo KUBO, AND MICHAEL M. WU Department of Neurobiology, Institute of Brain Research, School of Medicine, University of Tokyo, Tokyo, Japan Communicated by S. Hagiwara, March 6, 1986 ABSTRACT Electrophysiological properties of cerebellar tissue was dissociated by triturating with a fire-polished granule cells and synapses between granule and Purkinje cells Pasteur pipette in Ca-free Hanks' balanced salt solution were studied in dissociated cultures. Electrophysiological prop- containing 0.05% DNase and 12 mM MgSO4. The cells were erties of neurons and synapses in the mammalian central centrifuged at 150 x g at 40C and the pelleted cells were nervous system are best studied in dissociated cell cultures resuspended at a concentration of about 106 cells per ml in a because of good target cell visibility, control over the contents defined medium (9). One milliliter ofthe cell suspension from of the extracellular solution, and the feasibility of whole-cell newborn rats was plated first in a Petri dish (3.5 cm in patch electrode recording, which has been a powerful tech- diameter) containing several pieces ofheat-sterilized, poly(L- nique in analyzing biophysical properties of ionic channels in lysine)-coated coverslips, and then 1 ml of fetal cell suspen- small cells. We have applied this whole-cell recording technique sion was added. One-half of the culture medium was ex- to cultured cerebellar granule cells whose electrophysiological changed with fresh medium once a week. -
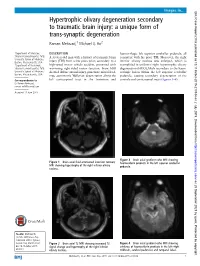
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

Closed-Loop Neuromodulation Restores Network Connectivity And
RESEARCH ARTICLE Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury Patrick D Ganzer1,2*, Michael J Darrow1, Eric C Meyers1,2, Bleyda R Solorzano2, Andrea D Ruiz2, Nicole M Robertson2, Katherine S Adcock3, Justin T James2, Han S Jeong2, April M Becker4, Mark P Goldberg4, David T Pruitt1,2, Seth A Hays1,2,3, Michael P Kilgard1,2,3, Robert L Rennaker II1,2,3* 1Erik Jonsson School of Engineering and Computer Science, The University of Texas at Dallas, Richardson, United States; 2Texas Biomedical Device Center, Richardson, United States; 3School of Behavioral Brain Sciences, The University of Texas at Dallas, Richardson, United States; 4Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, United States Abstract Recovery from serious neurological injury requires substantial rewiring of neural circuits. Precisely-timed electrical stimulation could be used to restore corrective feedback mechanisms and promote adaptive plasticity after neurological insult, such as spinal cord injury (SCI) or stroke. This study provides the first evidence that closed-loop vagus nerve stimulation (CLV) based on the synaptic eligibility trace leads to dramatic recovery from the most common forms of SCI. The addition of CLV to rehabilitation promoted substantially more recovery of forelimb function compared to rehabilitation alone following chronic unilateral or bilateral cervical SCI in a rat model. Triggering stimulation on the most successful movements is critical to maximize recovery. CLV enhances recovery by strengthening synaptic connectivity from remaining motor *For correspondence: networks to the grasping muscles in the forelimb. The benefits of CLV persist long after the end of [email protected] stimulation because connectivity in critical neural circuits has been restored. -

Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: a Study Using a High-Resolution Semiconductor PET System
Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: A Study Using a High-Resolution Semiconductor PET System Kenji Hirata1,2, Naoya Hattori3, Wataru Takeuchi4, Tohru Shiga1, Yuichi Morimoto4, Kikuo Umegaki5, Kentaro Kobayashi1, Osamu Manabe1, Shozo Okamoto1, and Nagara Tamaki1 1Department of Nuclear Medicine, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 2Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA, Los Angeles, California; 3Department of Molecular Imaging, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 4Research and Development Group, Hitachi Ltd., Tokyo, Japan; and 5Division of Quantum Science and Engineering, Faculty of Engineering, Hokkaido University, Sapporo, Japan (1). The rubrospinal tract mainly controls limb musculature, whereas The red nucleus (RN) is a pair of small gray matter structures located the pyramidal tract can act on the whole musculature (1). A recent in the midbrain and involved in muscle movement and cognitive anatomic study indicated that a significant amount of rubrospinal tract functions. This retrospective study aimed to investigate the metabo- is present in the human brain (2). Because neuronal activity of the RN lism of human RN and its correlation to other brain regions. Methods: in Parkinson disease is known to be increased during passive and We developed a high-resolution semiconductor PET system to image voluntary movements (3), the RN may play a role in the coordination small brain structures. Twenty patients without neurologic disorders of muscular movement. Cognitive symptoms, such as intellectual underwent whole-brain scanning after injection of 400 MBq of fatigability, decreased verbal fluency, and discrete memory impair- 18F-FDG. -

Compensatory Sprouting and Impulse Rerouting After Unilateral Pyramidal Tract Lesion in Neonatal Rats
The Journal of Neuroscience, September 1, 2000, 20(17):6561–6569 Compensatory Sprouting and Impulse Rerouting after Unilateral Pyramidal Tract Lesion in Neonatal Rats Werner J. Z’Graggen, Karim Fouad, Olivier Raineteau, Gerlinde A. S. Metz, Martin E. Schwab, and Gwendolyn L. Kartje Brain Research Institute, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8057 Zurich, Switzerland After lesions of the developing mammalian CNS, structural plas- tigate possible new functional connections from the motor cortex ticity and functional recovery are much more pronounced than in of the pyramidotomy side to the periphery. In rats lesioned as the mature CNS. We investigated the anatomical reorganization adults, stimulation of the motor cortex ipsilateral to the pyra- of the corticofugal projections rostral to a unilateral lesion of the midotomy never elicited EMG activity. In contrast, in P2 lesioned corticospinal tract at the level of the medullary pyramid (pyra- rats bilateral forelimb EMGs were found. EMG latencies were midotomy) and the contribution of this reorganization and other comparable for the ipsilateral and contralateral responses but descending systems to functional recovery. were significantly longer than in unlesioned animals. Transient Two-day-old (P2) and adult rats underwent a unilateral pyra- inactivation of both red nuclei with the GABA receptor agonist midotomy. Three months later the corticofugal projections to the muscimol led to a complete loss of these bilateral movements. red nucleus and the pons were analyzed; a relatively large num- Movements and EMGs reappeared after wash-out of the drug. ber of corticorubral and corticopontine fibers from the lesioned These results suggest an important role of the red nucleus in the side had crossed the midline and established an additional con- tralateral innervation of the red nucleus and the pons. -

Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study
Original Article J. Phys. Ther. Sci. 22: 7–10, 2010 Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study SUNG HO JANG, MD1), JI HEON HONG, PT, MS1), YONG HYUN KWON, PT, PhD2) 1)Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 2)Department of Physical Therapy, Yeungnam College of Science & Technology: 1737, Daemyung 7-Dong, Namgu, Daegu, 705-703, Republic of Korea. TEL +82 53-650-9702, FAX: +82 53-629-5048 Abstract. [Purpose] Little is known about the rubro-olivary tract (ROT) in the human brain. We attempted to identify the ROT using diffusion tensor tractography (DTT). [Subjects and Methods] We obtained ROT data from 11 healthy subjects with no history of neurological disorder. For tracking of the ROT, a seed region of interest (ROI) was selected in the red nucleus, and a target ROI was found in the inferior olive of each subject. [Results] The ROT, originated in the red nucleus, and passed laterally to the decussation of the superior cerebellar peduncle in the lower midbrain. In the pons, it descended through the area adjacent to the medial lemnicus in the posterior direction. Within the medulla, the ROT ended in the inferior olive, which was located lateral to the medial lemnicus and posterior to the pyramid. [Conclusion] We identified the ROT in the human brain using DTT. These results will be informative for research into the ROT in the human brain. Key words: Diffusion tensor image, Red nucleus, Inferior olive (This article was submitted Jul. 24, 2009, and was accepted Sep.