Extra-Adrenal Pheochromocytoma: Diagnosis and Management
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
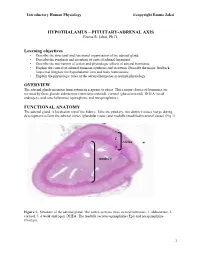
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Radiotherapy of Malignant Pheochromocytoma—A Case Report
Case Report Page 1 of 7 Radiotherapy of malignant pheochromocytoma—a case report Chi-Yuan Yeh Department of Radiation Oncology, Tungs’ Taichung Metroharbor Hospital, Taichung, Taiwan Correspondence to: Department of Radiation Oncology, Tungs’ Taichung Metroharbor Hospital, No.699, Sec. 8, Taiwan Blvd., Taichung City 435, Taiwan. Email: [email protected]. Abstract: Pheochromocytomas (PCC) are rare tumors with an estimated incidence of 0.4 to 9.5 cases per 1 million per year. About 5–26% of PCC are malignant and presents with metastasis, for which there is currently no effective therapy. The treatment of choice is for PCC is radical surgery to reduce tumor burden, to provide symptomatic relief of catecholamine excess although complete eradication of the lesions is often not feasible. A number of case reports have been published on the role of radiotherapy for the treatment of PCC. Here we present a 53-year-old male stage III malignant PCC patient who received postoperative adjuvant radiotherapy. A review of current literature is also presented. Keywords: Radiotherapy; pheochromocytoma (PCC); malignant pheochromocytoma; hypertension Received: 12 December 2018; Accepted: 12 August 2019; Published: 27 August 2019. doi: 10.21037/tro.2019.08.02 View this article at: http://dx.doi.org/10.21037/tro.2019.08.02 Introduction reaction confirmed PCC. The term PCC was derived from the Greek words Pheochromocytomas (PCC) and paragangliomas (PGL) phaios (“dusky”), chroma (“color”), and cytoma (“tumor”). are rare catecholamine-secreting tumors that arise from The dark staining reaction of PCC tumor was caused by the chromaffin cells of the adrenal medulla and the sympathetic oxidation of intracellular catecholamines when stained with ganglia respectively. -
Avian Adrenal Medulla: Cytomorphology and Function
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Publications of the IAS Fellows Volume 45(1-4):1-11, 2001 Acta Biologica Szegediensis http://www.sci.u-szeged.hu/ABS REVIEW ARTICLE Avian adrenal medulla: cytomorphology and function Asok Ghosh, Stephen W. Carmichael1*, Monisha Mukherjee Department of Zoology, University of Calcutta, Calcutta, India, 1Department of Anatomy, Mayo Clinic/Foundation, Rochester, Minnesota, USA ABSTRACT The purpose of this review is to explore the world literature on the avian adrenal KEY WORDS medulla from the last 20 years. Unlike the mammalian adrenal medulla, the adrenal gland in adrenal medulla birds has chromaffin cells mixed with cortical cells. Studies have investigated the ultrastructure birds (both transmission and scanning electron microscopy), biochemistry, and physiology (partic- morphology ularly interactions with other endocrine glands) of the avian adrenal medulla. Although function progress has been made, it is apparent that research on the avian adrenal medulla still lags behind work on the mammalian organ. Acta Biol Szeged 45(1-4):1-11 (2001) The adrenal glands of birds, like those in mammals, are in the adrenal medulla. This profound variation of medullary paired yellow- or orange-colored pear- or triangle-shaped E/NE ratio in birds suggests a distinct evolutionary pattern glands that are next to the kidneys. The intermingling nature (Ghosh 1977, 1980). The avian phylogeny used in this study of cortical and medullary components constitutes a major was essentially based on palaeontological evidences (Grego- characteristic of avian adrenal medulla (Vestergaard and ry 1957). We feel that our “claim” of hormonal taxonomy is Willeberg 1978). -

Endocrine Tumors – Adrenal Medulla
Endocrine Tumors – Adrenal Medulla 803-808-7387 www.gracepets.com These notes are provided to help you understand the diagnosis or possible diagnosis of cancer in your pet. For general information on cancer in pets ask for our handout “What is Cancer”. Your veterinarian may suggest certain tests to help confirm or eliminate diagnosis, and to help assess treatment options and likely outcomes. Because individual situations and responses vary, and because cancers often behave unpredictably, science can only give us a guide. However, information and understanding for tumors in animals is improving all the time. We understand that this can be a very worrying time. We apologize for the need to use some technical language. If you have any questions please do not hesitate to ask us. What are the adrenal glands? The adrenal glands are located close to the kidneys. They are part of the body’s endocrine system, the glands of which also include the pituitary (in the brain), thyroid, parathyroid and Islets of Langerhans in the pancreas. Endocrine glands produce specialized chemicals called “hormones”. These regulate and integrate many activities to maintain internal stability of the body. The hormones pass directly into the blood to affect target cells elsewhere. Hormones are also produced by many cells in other tissues. Each of the two adrenal glands has two parts. The outer part (cortex) is controlled by a hormone (adrenocorticotrophic hormone, ACTH) from the pituitary gland. The cortex produces steroid hormones of several types. The inner part (medulla) of the adrenal gland originates from the same cells that in the embryo form the nervous system, and therefore not surprisingly it produces neuroendocrine hormones with effects similar to those of the sympathetic nervous system. -

Chapter 20: Endocrine System
EndocrineEndocrine SystemSystem Modified by M. Myers 1 TheThe EndocrineEndocrine SystemSystem 2 EndocrineEndocrine GlandsGlands z The endocrine system is made of glands & tissues that secrete hormones. z Hormones are chemicals messengers influencing a. metabolism of cells b. growth and development c. reproduction, d. homeostasis. 3 HormonesHormones Hormones (chemical messengers) secreted into the bloodstream and transported by blood to specific cells (target cells) Hormones are classified as 1. proteins (peptides) 2. Steroids 4 HormoneHormone ClassificationClassification z Steroid Hormones: – Lipid soluble – Diffuse through cell membranes – Endocrine organs z Adrenal cortex z Ovaries z Testes z placenta 5 HormoneHormone ClassificationClassification z Nonsteroid Hormones: – Not lipid soluble – Received by receptors external to the cell membrane – Endocrine organs z Thyroid gland z Parathyroid gland z Adrenal medulla z Pituitary gland z pancreas 6 HormoneHormone ActionsActions z “Lock and Key” approach: describes the interaction between the hormone and its specific receptor. – Receptors for nonsteroid hormones are located on the cell membrane – Receptors for steroid hormones are found in the cell’s cytoplasm or in its nucleus 7 http://www.wisc- online.com/objects/index_tj.asp?objID=AP13704 8 EndocrineEndocrine SystemSystem z There is a close assoc. b/w the endocrine & nervous systems. z Hormone secretion is usually controlled by either negative feedback or antagonistic hormones that oppose each other’s actions 9 HypothalamusHypothalamus 1. regulates the internal environment through the autonomic system 2. controls the secretions of the pituitary gland. 10 HypothalamusHypothalamus && PituitaryPituitary GlandGland posteriorposterior pituitary/pituitary/ anterioranterior pituitarypituitary 11 PosteriorPosterior PituitaryPituitary The posterior pituitary secretes zantidiuretic hormone (ADH) zoxytocin 12 13 14 AnteriorAnterior pituitarypituitary glandgland 1. -

Surgical Indications and Techniques for Adrenalectomy Review
THE MEDICAL BULLETIN OF SISLI ETFAL HOSPITAL DOI: 10.14744/SEMB.2019.05578 Med Bull Sisli Etfal Hosp 2020;54(1):8–22 Review Surgical Indications and Techniques for Adrenalectomy Mehmet Uludağ,1 Nurcihan Aygün,1 Adnan İşgör2 1Department of General Surgery, Sisli Hamidiye Etfal Training and Research Hospital, Istanbul, Turkey 2Department of General Surgery, Bahcesehir University Faculty of Medicine, Istanbul, Turkey Abstract Indications for adrenalectomy are malignancy suspicion or malignant tumors, non-functional tumors with the risk of malignancy and functional adrenal tumors. Regardless of the size of functional tumors, they have surgical indications. The hormone-secreting adrenal tumors in which adrenalectomy is indicated are as follows: Cushing’s syndrome, arises from hypersecretion of glucocorticoids produced in fasciculata adrenal cortex, Conn’s syndrome, arises from an hypersecretion of aldosterone produced by glomerulosa adrenal cortex, and Pheochromocytomas that arise from adrenal medulla and produce catecholamines. Sometimes, bilateral adre- nalectomy may be required in Cushing's disease due to pituitary or ectopic ACTH secretion. Adenomas arise from the reticularis layer of the adrenal cortex, which rarely releases too much adrenal androgen and estrogen, may also develop and have an indication for adrenalectomy. Adrenal surgery can be performed by laparoscopic or open technique. Today, laparoscopic adrenalectomy is the gold standard treatment in selected patients. Laparoscopic adrenalectomy can be performed transperitoneally or retroperitoneoscopi- cally. Both approaches have their advantages and disadvantages. In the selection of the surgery type, the experience and habits of the surgeon are also important, along with the patient’s characteristics. The most common type of surgery performed in the world is laparoscopic transabdominal lateral adrenalectomy, which most surgeons are more familiar with. -

The Endocrine System
4/12/2016 Adrenal Glands Image From: http://www.hawaiilife.com/articles/2012/03/good-news-vacation-rental-owners/ 70 Copyright © 2009 Pearson Education, Inc Figure 10.14a Adrenal Glands Adrenal Adrenal cortex Adrenal Glands gland • Mineralocorticoids • Gonadocorticoids • Glucocorticoids • Controlled by both nerves and hormones – Adrenal medulla Adrenal Medulla • Epinephrine • Controlled by nerves from the hypothalamus • Norepinephrine – Adrenal Cortex • Controlled by ACTH (a hormone) secreted by the anterior pituitary gland (b) A section through the adrenal gland reveals two regions, the outer adrenal cortex and the inner adrenal medulla. These regions secrete different hormones. 73 Copyright © 2009 Pearson Education, Inc Figure 10.14b Adrenal Glands Adrenal Glands • Adrenal Medulla • Adrenal Cortex – Epinephrine – 2 types of hormones secreted • Increases blood pressure • Mineralocorticoids • Increases heart rate • Glucocorticoids • Increases blood glucose levels 74 Copyright © 2009 Pearson Education, Inc 75 Copyright © 2009 Pearson Education, Inc 1 4/12/2016 Adrenal Glands - Cortex Adrenal Glands - Cortex • Mineralocorticoids • Aldosterone – Example: Aldosterone – Promotes renal absorption of Na+ and renal – Mineral homeostasis excretion of K+ – Water balance – Increased blood pressure • Target – Kidneys 76 Copyright © 2009 Pearson Education, Inc 77 Copyright © 2009 Pearson Education, Inc Adrenal Glands - Cortex Adrenal Glands - Cortex • Glucocorticoids • Cortisol – Example: Cortisol – Effects glucose homeostasis – Act on the liver to -

Adrenal and Paraganglia Tumors Adrenal Cortical Tumors IHC: (+) SF1, Inhibin, Melan-A, Calretinin, Synaptophysin, (-) Chromogranin, Cytokeratin, S100
Last updated: 11/11/2020 Prepared by Kurt Schaberg Adrenal and Paraganglia Tumors Adrenal Cortical Tumors IHC: (+) SF1, Inhibin, Melan-A, Calretinin, Synaptophysin, (-) Chromogranin, Cytokeratin, S100. Often variable!! Adrenal Cortical Adenoma Benign. Very common. Often incidentally identified. Usually unilateral solitary masses with atrophic background adrenal gland. Tumor cells can be lipid-rich (clearer) or lipid-poor (pinker) arranged in nests and cords separated by abundant vasculature. Occasional lipofuscin pigment. Nuclei generally small and round (occasional extreme “endocrine atypia” is common). Low/no mitotic activity. Intact reticulin framework. On a spectrum with and may hard to differentiate from hyperplastic nodules, which is more often multinodular (background hyperplasia) and bilateral. Can be non-functional (85%) or functional (15%). Associated with MEN1, FAP, Carney Complex, among Aldosterone-producing→ “Conn syndrome”→ others… hypertension and hypokalemia If aldosterone-secreting adenoma is treated with Cortisol-producing→ (ACTH-independent) spironolactone→ “spironolactone bodies” (below) “Cushing Syndrome” → central obesity, moon face, hirsutism, poor healing, striae Sex-hormone-producing→ Rare (more common in carcinomas). Symptoms depend on hormone/sex (virilization or feminization) Adrenal Cortical Carcinoma Malignant. Most common in older adults. Can present with an incidental unilateral mass or with an endocrinopathy (see above). Solid, broad trabeculae, or large nested growth (more diffuse, and larger groups than in adenomas) Thick fibrous capsule with occasional fibrous bands. Frequent tumor necrosis. Frequent vascular or capsular invasion. Increased mitotic activity. Variants: Oncocytic, Myxoid, Sarcomatoid Mostly sporadic, but can be associated with Lynch Syndrome and Li-Fraumeni Syndrome Distinguishing between an Adrenal Cortical Adenoma vs Carcinoma Weiss Criteria: Weiss Criteria (≥3 = Malignant) Most widely used system, but doesn’t work as well High nuclear grade (based of Fuhrman criteria) in borderline cases or variants. -

Haemorrhagic Retroperitoneal Paraganglioma
Yang et al. BMC Surg (2020) 20:304 https://doi.org/10.1186/s12893-020-00953-y CASE REPORT Open Access Haemorrhagic retroperitoneal paraganglioma initially manifesting as acute abdomen: a rare case report and literature review Yanliang Yang1, Guangzhi Wang2, Haofeng Lu1, Yaqing Liu2, Shili Ning2† and Fuwen Luo2*† Abstract Background: Paragangliomas (PGLs) are extremely rare neuroendocrine tumours arising from extra-adrenal chro- mafn cells. PGLs are clinically rare, difcult to diagnose and usually require surgical intervention. PGLs mostly present catecholamine-related symptoms. We report a case of Acute abdomen as the initial manifestation of haemorrhagic retroperitoneal PGL. There has been only one similar case reported in literature. Case presentation: We present a unique case of a 52-year-old female with acute abdomen induced by haemor- rhagic retroperitoneal PGL. The patient had a 5-h history of sudden onset of serve right lower quadrant abdominal pain radiating to the right fank and right lumbar region. Patient had classic symptoms of acute abdomen. Abdominal ultrasound revealed a large abdominal mass with a clear boundary. A Computed Tomography Angiography (CTA) of superior mesenteric artery was also performed to in the emergency department. The CTA demonstrated a large retro- peritoneal mass measured 9.0 7.3 cm with higher density inside. A provisional diagnosis of retroperitoneal tumour with haemorrhage was made. ×The patient received intravenous fuids, broad-spectrum antibiotics and somatostatin. On the 3rd day of admission, her abdominal pain was slightly relieved, but haemoglobin decreased from 10.9 to 9.4 g/ dL in 12 h suggesting that there might be active bleeding in the abdominal cavity. -

Adrenal Medulla
Adrenal medulla Objectives: ❖ Summarize the actions of adrenal androgens. ❖ Describe the causes and major manifestations of hyperadrenocorticism and Hypoadrenocorticism. ❖ Describe circumstances in which catecholamines are released from the adrenal gland. ❖ List the major actions of catecholamines. Done by : ➔ Team leader: Rahaf AlShammari, Abdulelah AlDossari ➔ Team members: ◆ Esraa AlNazzawi, Renad AlMogren ◆ Abdulmajeed AlWardi, Abdulelah AlSaeed Colour index: ◆ Anas AlSaif, Saif AlMeshari ● Important ◆ Laila AlSabbagh, Fatimah Blasharaf ● ;) ◆ Renad AlSwailmy, Wejdan AlShamery ● Extra َوأَن ﻟﱠﯾْسَ ﻟِ ِْﻺَﻧﺳِﺎن إِﱠﻻ ﻣَﺎ َﺳَﻌٰﻰ Adrenal medulla ● The adrenal medulla is the inner part or core of each adrenal gland. ● It is considered as part of sympathetic nervous system. ● It synthesizes and secretes catecholamines from Tyrosine: Adrenaline (epinephrine) -- 80% of the secretion. Noradrenaline (norepinephrine) -- 20 % of the secretion. Small amount of dopamine ● They are released from chromaffin cells ● Secretion of these hormones causes: Blood to be diverted to the brain, heart, and skeletal muscle. ● Epinephrine is the more potent stimulator of the heart and metabolic activities ● Norepinephrine is more influential on peripheral vasoconstriction and blood pressure ● High levels of cortisol that drain into the medulla from the adrenal cortex induce expression of the enzyme phenylethanolamine N-methyltransferase (PNMT), which converts norepinephrine to epinephrine Role of the adrenal medullary hormones 1. Enhance the effects of the 2. Prepare