Elemental and Molecular Profiling of Illicit Tobacco
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Take a Stand Against Illegal Tobacco
Take a stand against Illegal Tobacco The current state of play Illegal tobacco sales in the UK continue to undermine legitimate business whilst providing criminals with huge profits. The continued availability of cheap illegal tobacco in communities across the country undermines attempts to prevent underage people accessing tobacco as illegal tobacco sellers do not care who they sell to. The smuggling of tobacco is a multibillion pound industry for criminals. The high level of tax in the UK, the large difference in the tax rates between the UK and other countries, and the openness of international borders creates the perfect environment for criminals to operate. Criminal organisations and opportunist smugglers are targeting the lucrative UK tobacco market. HM Revenue & Customs' 2012/13 estimates show that revenue theft could be as much as £2.9 billion.1 For a criminal, tobacco smuggling is relatively easy. Tobacco products come in small packages, are highly valuable and easy to transport; whilst the risks of detection, prosecution and conviction, though improving, are low relative to the huge profits. For example, a single container of illegal cigarettes can provide criminals with a profit of up to £2 million.2 It's clear more needs to be done! Did you know? HM Revenue & Customs estimates show that revenue A single container of illegal theft could be as cigarettes can provide criminals much as £2.9 billion1 with a profit of up to £2 million Illegal Tobacco Explained What is NUKDP? Non-UK duty paid (NUKDP) is a catch-all phrase used to describe cigarettes and roll your own tobacco (RYO) found in the UK that has not incurred UK taxes. -

Tobacco Control Survey, England 2019-2020
www.tradingstandards.uk TOBACCO CONTROL SURVEY ENGLAND 2019/20 Jane MacGregor, MacGregor Consulting Limited for the Chartered Trading Standards Institute 18 1 Tobacco Control Survey, England 2019/2020: A Report of Trading Standards Service Activity CONTENTS Summary 4 Premises where products were Figure 7: Percentage of test purchase Introduction 4 non-compliant with SPoT 26 operations resulting in illegal sales Context 5 Actions taken 27 between 2008/09 and 2019/20 12 Methodology 5 Actions taken UAS tobacco 28 Figure 8: Underage sales: tobacco - Actions taken UAS NIPs 28 proportion of test purchase operations Tobacco control activities 6 by type of premises 13 Actions taken illicit tobacco 29 Priority given to tobacco control activities 6 Figure 9: Underage sales: nicotine Tobacco control activities 8 Actions taken TRPRs tobacco 29 inhaling products - total number of test Underage sales 9 Actions taken TRPRs NIPs 30 purchase operations and sales made 15 Tobacco products 10 Actions taken SPoT 31 Figure 10: Underage sales: nicotine Complaints and enquiries received 10 Conclusion 31 inhaling products - percentage visits Complaints and enquiries received by Underage sales: tobacco products 31 resulting in illegal sale 15 premises type 10 Underage sales: NIPs 32 Figure 11: Underage sales: nicotine Test purchase operations for tobacco 11 Illicit tobacco products 32 inhaling products - proportion of test purchase operations by type of premises 16 Number of test purchase operations Tobacco and Related Products resulting in illegal sale 11 Regulations -

Selling Tobacco, Electronic Cigarettes and E-Liquids the Consequences
Selling Tobacco, Electronic Cigarettes and E-Liquids • You must be registered with the Scottish Government to sell tobacco products, electronic cigarettes or e-liquids by retail. Register online at: www.tobaccoregisterscotland.org/ • It is an offence to supply a tobacco product, cigarette papers, electronic cigarettes or e-liquids to anyone under 18 (even if they claim it is for an adult) • It is an offence for any person under 18 to buy a tobacco product or cigarette papers. • It is an offence for any person over 18 to knowingly buy or attempt to buy a tobacco product or cigarette papers on behalf of someone under 18. • Cigarettes must only be sold in packs of 20 or more. It is illegal to sell single cigarettes. • The sale of cigarettes via a vending machine for use by customers is prohibited. The Consequences of Not Complying with the Law: There are serious consequences for those who do not comply with tobacco sales legislation. Trading Standards Officers have powers to issue Fixed Penalty Notices for offences such as selling to someone aged under 18, not being a registered seller, and not displaying the required notice (see below). The fixed penalty has been set at £200. This will increase by £200 for every offence committed within a two year period. It should be noted that a fixed penalty can be issued to either a sales assistant, the owner of the premises, or both. If a retailer is found to be in breach of tobacco sales legislation three times within a two year period, the local authority can apply to the courts to have the retailer banned from selling tobacco. -

Assessing the Impacts of Revising the Tobacco Products Directive Study to Support a DG SANCO Impact Assessment
CHILDREN AND FAMILIES The RAND Corporation is a nonprofit institution that helps improve policy and EDUCATION AND THE ARTS decisionmaking through research and analysis. ENERGY AND ENVIRONMENT HEALTH AND HEALTH CARE This electronic document was made available from www.rand.org as a public INFRASTRUCTURE AND service of the RAND Corporation. TRANSPORTATION INTERNATIONAL AFFAIRS LAW AND BUSINESS NATIONAL SECURITY Skip all front matter: Jump to Page 16 POPULATION AND AGING PUBLIC SAFETY SCIENCE AND TECHNOLOGY Support RAND TERRORISM AND Browse Reports & Bookstore HOMELAND SECURITY Make a charitable contribution For More Information Visit RAND at www.rand.org Explore RAND Europe View document details Limited Electronic Distribution Rights This document and trademark(s) contained herein are protected by law as indicated in a notice appearing later in this work. This electronic representation of RAND intellectual property is provided for non-commercial use only. Unauthorized posting of RAND electronic documents to a non-RAND Web site is prohibited. RAND electronic documents are protected under copyright law. Permission is required from RAND to reproduce, or reuse in another form, any of our research documents for commercial use. For information on reprint and linking permissions, please see RAND Permissions. This product is part of the RAND Corporation technical report series. Reports may include research findings on a specific topic that is limited in scope; present discussions of the methodology employed in research; provide literature reviews, survey instru- ments, modeling exercises, guidelines for practitioners and research professionals, and supporting documentation; or deliver preliminary findings. All RAND reports un- dergo rigorous peer review to ensure that they meet high standards for research quality and objectivity. -

Điểm Khó Khăn Đối Với Chúng Tôi, Vì Nhu Cầu Về Thuốc Lá Đã Giảm Do Một Số Đợt Tăng Thuế Tiêu Thụ Đặc Biệt
ĐĐIIỂỂMM TTHHUUỐỐCC LLÁÁ TTHHẾẾ GGIIỚỚII TTIINN 0033 NNÔÔNNGG DDÂÂNN && NNGGUUYYÊÊNN LLIIỆỆUU Thái Lan: Cơ quan độc quyền thuốc lá sẽ giúp nông dân chuyển sang trồng cây cần sa 24/02 - Với doanh số bán thuốc lá dự kiến sẽ giảm do thuế tăng mạnh, Cơ quan Thuốc lá Thái Lan có kế hoạch giúp nông dân thuốc lá chuyển sang trồng cây cần sa. Giám đốc cơ quan này, Daonoi Suttiniphapunt, cho biết mức tăng thuế tiêu thụ đặc biệt sẽ được áp dụng vào tháng 10 tới sẽ khiến giá một bao thuốc lá tăng từ 30 - 40% lên ít nhất là 93 Baht. Điều đó sẽ dẫn đến sự sụt giảm trong tiêu dùng, bà này dự đoán. Chính quyền sẽ chống thuốc lá tự quấn, có mức thuế thấp hơn. Dự kiến 19 tỷ điếu thuốc lá sẽ được sản xuất trong năm nay, khối lượng như năm ngoái, nhưng lợi nhuận có thể sẽ thu hẹp còn 400 triệu hoặc 500 triệu Baht so với 900 triệu Baht năm ngoái. Daonoi cho biết chính quyền sẽ hỗ trợ nông dân thuốc lá nếu họ muốn chuyển sang cây cần sa. Việc trồng loại cây này đã được hợp pháp hóa cho các mục đích y tế. (The Nation) Philippines: Lời đề nghị trợ giúp cho nông dân 11/02 - Nếu việc tăng thuế “tội lỗi” tiếp theo được thúc đẩy, chính phủ nên giúp nông dân thuốc lá và các nhà bán lẻ nhỏ đối phó với việc doanh thu có khả năng sụt giảm mà họ sẽ phải chịu, theo một câu chuyện trong tờ báo The Philippines Star trích lời thống đốc tỉnh Ilocos Norte, Imee Marcos. -

The Difference Between Recommended Retail Price And
Running head: RRP and standardised packaging 1 Title: The difference between Recommended Retail Price and Sales Price for tobacco products 2 in independent and convenience (small) retailers before and after the introduction of 3 standardised tobacco packaging in the United Kingdom. 4 5 Authors 6 Nathan Critchlow1 7 Martine Stead1 8 Crawford Moodie1,2 9 Kathryn Angus1 10 Douglas Eadie1 11 Anne Marie MacKintosh1,2 12 13 Affiliations: 14 1 Institute for Social Marketing, UK Centre for Tobacco and Alcohol Studies, Faculty of Health 15 Sciences and Sport, University of Stirling, FK9 4LA, Scotland. 16 17 2 Centre for Tobacco Control Research, Institute for Social Marketing, Faculty of Health 18 Sciences and Sport, University of Stirling, FK9 4LA, Scotland. 19 20 Corresponding author: Nathan Critchlow, Institute for Social Marketing, Faculty of Health 21 Sciences and Sport, University of Stirling, Stirling, FK9 4LA, Scotland. E-mail: 22 [email protected]. Tel: +44(0)1786 467382. 23 24 Word count: 3,603 25 Table count: 5 26 Supplementary tables: 1 27 28 This article has been accepted for publication in Tobacco Control following peer review, 29 and the Version of Record can be accessed online at 30 https://doi.org/10.1136/tobaccocontrol-2018-054409 31 32 1 Running head: RRP and standardised packaging 1 The difference between Recommended Retail Price and Sales Price in independent and 2 convenience (small) retailers before and after the introduction of standardised tobacco 3 packaging in the United Kingdom. 4 5 ABSTRACT 6 Aim: Recommended Retail Price (RRP) is a marketing strategy used by tobacco companies to 7 maintain competitiveness, communicate product positioning, and drive sales. -

Japan Tobacco International (JTI) 2017 Third Quarter Results
Tokyo, November 1, 2017 Japan Tobacco Inc. Japan Tobacco International (JTI) 2017 Third Quarter Results Earnings growth remains on track for full year (billions of units, millions of US$) Jul-Sep Change Jan-Sep Change vs. prior vs. prior 2017 2016 year 2017 2016 year 1 Total shipment volume 104.8 104.5 0.3% 298.0 304.2 -2.0% 1 GFB shipment volume 76.0 75.5 0.5% 216.8 216.3 0.2% 2 Core revenue 2,754 2,823 -2.4% 7,895 8,062 -2.1% Core revenue at constant FX 2,717 2,823 -3.7% 7,941 8,062 -1.5% Adjusted operating profit 1,009 897 12.4% 2,747 2,626 4.6% Adjusted operating profit at constant FX 982 897 9.4% 2,868 2,626 9.2% Highlights 2017 third quarter results (July-September) • Adjusted operating profit at constant FX grew 9.4%, driven by cost optimization. On a reported basis, adjusted operating profit increased 12.4% supported by positive currency impacts. • Core revenue decreased 3.7% at constant FX due to negative volume and price/mix variance. Favorable currency movements resulted in reported core revenue decreasing 2.4%. • Total shipment volume grew 0.3% driven by the acquisition in the Philippines. Excluding this acquisition, total shipment volume declined 1.2% mainly due to industry volume contraction. • GFB shipment volume increased 0.5% driven by strong performance of Winston and Camel. • Investment continued in emerging products. Logic, our e-cigarette brand, was launched in Russia. Our tobacco vapor product3 Ploom TECH was introduced in Switzerland in July and was expanded to four US states in September under the brand name Vapeleaf by Logic4. -

TACKLING the SUPPLY of ILLICIT TOBACCO PRODUCTS in ENGLAND 2014 Commissioned by the Department of Health Tobacco Policy Team
OPERATION HENRY: TACKLING THE SUPPLY OF ILLICIT TOBACCO PRODUCTS IN ENGLAND 2014 Commissioned by the Department of Health Tobacco Policy Team Jane MacGregor, MacGregor Consulting Ltd for the TSI [email protected] Summary and Key Findings Operation Henry is the first large scale coordinated action by Trading Standards to tackle the local supply of illicit tobacco products across England The Operation was developed and managed by the Trading Standards Institute on behalf of the Department of Health. Tobacco detection dog search teams were provided by Wagtail UK Ltd Illicit tobacco products were available in every English region 81 local authority Trading Standards Services took part in Operation Henry; seizures occurred in 67 of those authorities The majority of seizures were made at small retailers, independent newsagents and off license premises The highest seizure rate was in the North East of England with 94% of premises searched revealing illicit tobacco products The lowest seizure rate was in London with 28% of premises searched revealing illicit tobacco products There were 1,855,976 genuine non UK duty paid cigarettes seized o The largest single seizure was 1.4 million sticks detected at a self- store facility in London There were 615, 540 illicit white cigarettes seized with Jin Ling , Pect / Fest, NZ Gold and Gold Mount brands reported most often o The largest single seizure of illicit whites was 87,770 sticks detected at a business storage unit in the East of England The total value of the seized tobacco products was £753,499 o £439,513.68 from the seizure of genuine non UK duty paid cigarettes o £145,766.03 from seizure of illicit white cigarettes o £92,395.52 from non UK duty paid hand rolling tobacco 1 MacGregor Consulting Ltd for the Trading Standards Institute Contents Summary and Key Findings .............................................................................................................................. -

A Systematic Review of Research Exploring the Response of Consumers, Retailers and Tobacco Companies to Standardised Packaging in the United Kingdom
A systematic review of research exploring the response of consumers, retailers and tobacco companies to standardised packaging in the United Kingdom Crawford Moodie Kathryn Angus Martine Stead Institute for Social Marketing, University of Stirling October 2019 Acknowledgements We would like to thank Dr. Alison O’Mara-Eves and Dr. Dylan Kneale from the EPPI-Centre, at the Institute of Education (University College London), for acting as independent external assessors to conduct critical appraisal of each of the studies included within this review. We would also like to thank authors of the included studies for sharing additional information about their studies with us and our colleagues in the field of tobacco control for alerting us to relevant ongoing studies. The work was undertaken by the authors as part of the Public Health Research Consortium. The Public Health Research Consortium is funded by the Department of Health Policy Research Programme. The views expressed in the publication are those of the authors and not necessarily those of the Department of Health. Information about the wider programme of the PHRC is available from http://phrc.lshtm.ac.uk/ Table of Contents What this study adds: .................................................................................................................. 5 Abstract ....................................................................................................................................... 6 Introduction ................................................................................................................................. -

School of Business and Economics
A Work Project presented as part of the requirements for the Award of a Master Degree in Management from the NOVA – School of Business and Economics A MARKET RESEARCH ON THE TOBACCO CONSUMERS IN PORTUGAL: WHAT EXPLAINS THE SUCCESS OF IQOS AND WILL IT SUSTAIN IN THE FUTURE? GABRIELA EDUARDA ROCHA BRAZ, 33617 A Project carried out on the Master in Management Program, under the supervision of: Lena Kemna Catherine da Silveira 03.01.2020 Abstract The tobacco industry has been facing several transformations as technology evolves and consumers become increasingly health concerned. Philip Morris International leveraged on this trend and introduced IQOS in Portugal, converting several smokers to their heat-not-burn technology. This Work Project analyses the viability of this product in the Portuguese market as well as the strategy behind its current success, through the comprehension of the complexity of the tobacco industry and segmentation and quantification of the tobacco consumers in Portugal. It is concluded that IQOS is an established brand in Portugal, with several loyal consumers due to its convenient distribution and marketing strategy, which can still further penetrate the market through early mover advantages in the Portuguese Reduced Risk Products market. Keywords: IQOS, Tobacco, Market Segmentation, Industry Analysis This work used infrastructure and resources funded by Fundação para a Ciência e a Tecnologia (UID/ECO/00124/2013, UID/ECO/00124/2019 and Social Sciences DataLab, Project 22209), POR Lisboa (LISBOA-01-0145-FEDER-007722 and Social Sciences DataLab, Project 22209) and POR Norte (Social Sciences DataLab, Project 22209). Contents 1. Introduction & Objectives ...........................................................................................................1 2. -

JTI UK's Response to Tobacco and Related Products Regulation And
Consultation on the Tobacco and Related Products Regulations 2016 and the Standardised Packaging of Tobacco Products Regulations 2015 Response submitted on behalf of JTI UK 19 March 2021 About JTI Japan Tobacco International (JTI) is part of the Japan Tobacco group of companies, a leading international tobacco and e-cigarette manufacturer. JTI has its UK headquarters in Weybridge, Surrey, and has a long-standing and significant presence in the UK, with a national distribution centre in Crewe and a nationwide field-based Sales force. Its brand portfolio includes Benson & Hedges, Silk Cut, Mayfair, Sovereign and Sterling, as well as a number of other tobacco products including roll-your-own tobacco (RYO), also known as hand-rolling tobacco (such as Amber Leaf), cigars (such as Hamlet) and pipe tobacco (such as Condor). JTI sells nicotine pouches under its brand, Nordic Spirit. JTI is also a major player in reduced risk products with its brand, Logic, and heated tobacco brand, Ploom. JTI’s UK excise contributions on its tobacco products amounted to around £5.5 billion in 2020. The Tobacco and Related Products Regulations 2016 Question 1: How far do you agree or disagree that the introduction of rotating combined (photo and text) health warnings on cigarette and hand rolling tobacco has encouraged smokers to quit? • Strongly agree • Agree • Neither agree or disagree • Disagree • Strongly disagree • Don’t know There is no credible evidence to suggest that the introduction of rotating combined (photo and text) health warnings on cigarettes and hand rolling tobacco has encouraged smokers to quit. Smoking rates were already in decline well before the introduction of such regulations with the most recent Smoking Toolkit Study showing that an estimated 14.8% of adults smoked in 2020, compared with a rate of 21.4% in 2010 and 18.0% in 2016 (1). -
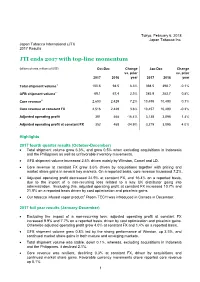
JTI 2017 Results
Tokyo, February 6, 2018 Japan Tobacco Inc. Japan Tobacco International (JTI) 2017 Results JTI ends 2017 with top-line momentum (billions of units, millions of US$) Oct-Dec Change Jan-Dec Change vs. prior vs. prior 2017 2016 year 2017 2016 year 1 Total shipment volume 100.5 94.5 6.3% 398.5 398.7 -0.1% 1 GFB shipment volume 69.1 67.4 2.5% 285.9 283.7 0.8% 2 Core revenue 2,603 2,429 7.2% 10,498 10,490 0.1% Core revenue at constant FX 2,516 2,429 3.6% 10,457 10,490 -0.3% Adjusted operating profit 391 468 -16.4% 3,138 3,095 1.4% Adjusted operating profit at constant FX 352 468 -24.9% 3,219 3,095 4.0% Highlights 2017 fourth quarter results (October-December) • Total shipment volume grew 6.3%, and grew 0.5% when excluding acquisitions in Indonesia and the Philippines as well as unfavorable inventory movements. • GFB shipment volume increased 2.5% driven mainly by Winston, Camel and LD. • Core revenue at constant FX grew 3.6% driven by acquisitions together with pricing and market share gains in several key markets. On a reported basis, core revenue increased 7.2%. • Adjusted operating profit decreased 24.9% at constant FX, and 16.4% on a reported basis, due to the impact of a non-recurring loss related to a key UK distributor going into administration. Excluding this, adjusted operating profit at constant FX increased 10.7% and 21.8% on a reported basis driven by cost optimization and price/mix gains.