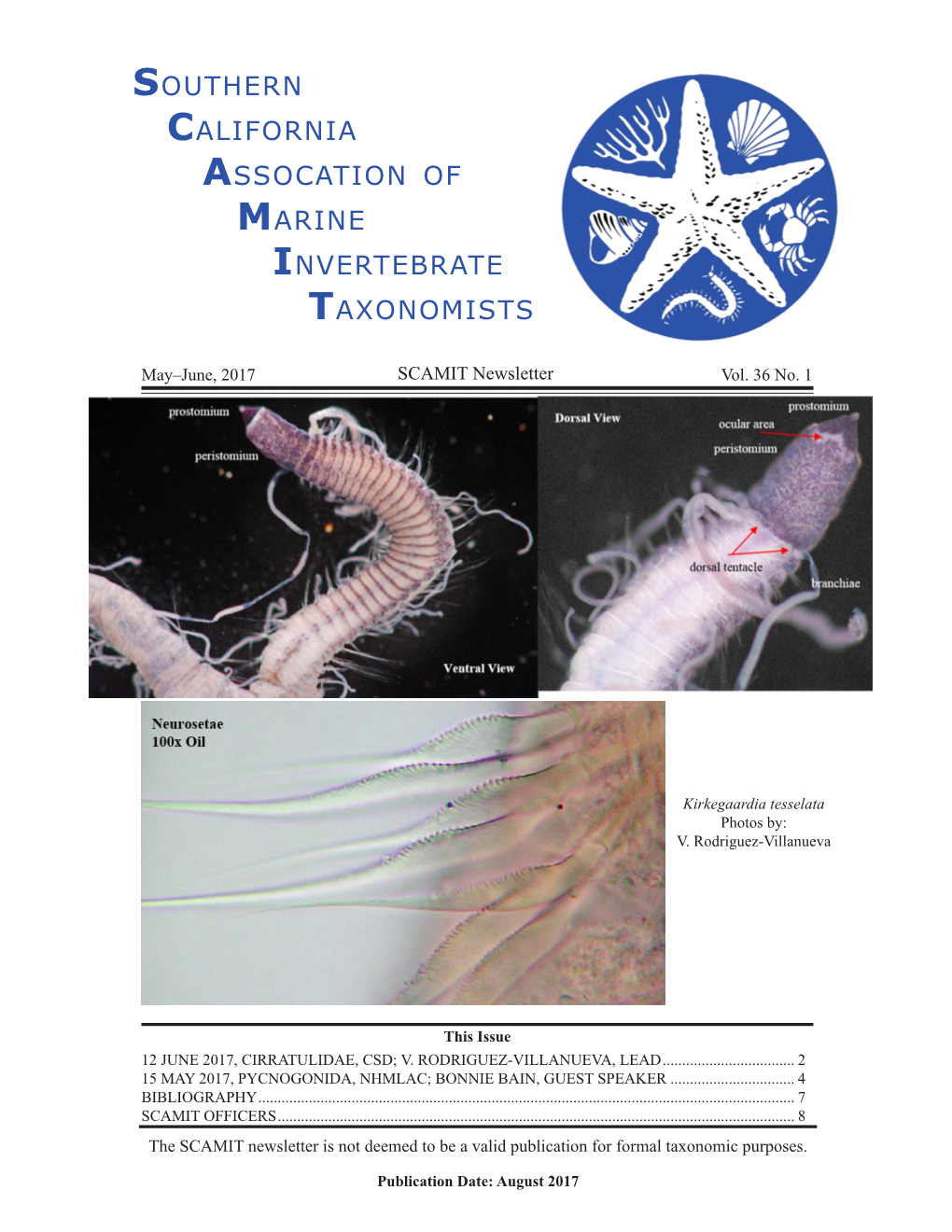
SCAMIT Newsletter Vol. 36 No. 1 2017 May/June
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanoreceptors in Early Developmental Stages of the Pycnogonida
UACE2019 - Conference Proceedings Mechanoreceptors in Early Developmental Stages of the Pycnogonida John A. Fornshell * a a U.S. National Museum of Natural History Department of Invertebrate Zoology Smithsonian Institution Washington, D.C. USA *Correspondence: [email protected]; Tel. (571) 426-2398 ABSTRACT Members of the phylum Arthropoda detect fluid flow and sound/particle vibrations using sensory organs called sensilla. These sensilla detect sound/particle vibrations in the boundary layer. In the present study, archived specimens from the United States National Museum of Natural History were examined in an effort to extend our knowledge of the presence of sensilla on the early post hatching developmental stages, first and second instars, of pycnogonids. In the work presented here we look at three families, four genera and ten species of early post hatching developmental stages of sea spiders. They are Family Ammotheidae, Achelia cuneatis Child, 1999, Ammothea allopodes Fry and Hedgpeth, 1969, Ammothea carolinensis Leach 1814, Ammothea clausi Pfeffer, 1889, Ammothea striata (Möbius, 1902), Family Nymphonidae, Nymphon grossipes (Fabricius, 1780), N. australe Hodgson, 1902, N. charcoti Bouvier, 1911, N. Tenellum (Sars, 1888) and Pycnogonidae, Pentapycnon charcoti Bouvier, 1910. Electron micrograph images of these species were used to identify and describe the sensilla present. Most body organs such as mouthparts, the eye tubercle, appendages and spines are proportionally much smaller in the early post hatching developmental stages compared to their size in the adults, while the sensilla are comparable in size and shape to those found on the adults. In the first instar of Pentapycnon charcoti sensilla are present, but not in the adult. -

Phylogenomic Resolution of Sea Spider Diversification Through Integration Of
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.31.929612; this version posted February 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Phylogenomic resolution of sea spider diversification through integration of multiple data classes 1Jesús A. Ballesteros†, 1Emily V.W. Setton†, 1Carlos E. Santibáñez López†, 2Claudia P. Arango, 3Georg Brenneis, 4Saskia Brix, 5Esperanza Cano-Sánchez, 6Merai Dandouch, 6Geoffrey F. Dilly, 7Marc P. Eleaume, 1Guilherme Gainett, 8Cyril Gallut, 6Sean McAtee, 6Lauren McIntyre, 9Amy L. Moran, 6Randy Moran, 5Pablo J. López-González, 10Gerhard Scholtz, 6Clay Williamson, 11H. Arthur Woods, 12Ward C. Wheeler, 1Prashant P. Sharma* 1 Department of Integrative Biology, University of Wisconsin–Madison, Madison, WI, USA 2 Queensland Museum, Biodiversity Program, Brisbane, Australia 3 Zoologisches Institut und Museum, Cytologie und Evolutionsbiologie, Universität Greifswald, Greifswald, Germany 4 Senckenberg am Meer, German Centre for Marine Biodiversity Research (DZMB), c/o Biocenter Grindel (CeNak), Martin-Luther-King-Platz 3, Hamburg, Germany 5 Biodiversidad y Ecología Acuática, Departamento de Zoología, Facultad de Biología, Universidad de Sevilla, Sevilla, Spain 6 Department of Biology, California State University-Channel Islands, Camarillo, CA, USA 7 Départment Milieux et Peuplements Aquatiques, Muséum national d’Histoire naturelle, Paris, France 8 Institut de Systématique, Emvolution, Biodiversité (ISYEB), Sorbonne Université, CNRS, Concarneau, France 9 Department of Biology, University of Hawai’i at Mānoa, Honolulu, HI, USA Page 1 of 31 bioRxiv preprint doi: https://doi.org/10.1101/2020.01.31.929612; this version posted February 2, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

South Carolina Department of Natural Resources
FOREWORD Abundant fish and wildlife, unbroken coastal vistas, miles of scenic rivers, swamps and mountains open to exploration, and well-tended forests and fields…these resources enhance the quality of life that makes South Carolina a place people want to call home. We know our state’s natural resources are a primary reason that individuals and businesses choose to locate here. They are drawn to the high quality natural resources that South Carolinians love and appreciate. The quality of our state’s natural resources is no accident. It is the result of hard work and sound stewardship on the part of many citizens and agencies. The 20th century brought many changes to South Carolina; some of these changes had devastating results to the land. However, people rose to the challenge of restoring our resources. Over the past several decades, deer, wood duck and wild turkey populations have been restored, striped bass populations have recovered, the bald eagle has returned and more than half a million acres of wildlife habitat has been conserved. We in South Carolina are particularly proud of our accomplishments as we prepare to celebrate, in 2006, the 100th anniversary of game and fish law enforcement and management by the state of South Carolina. Since its inception, the South Carolina Department of Natural Resources (SCDNR) has undergone several reorganizations and name changes; however, more has changed in this state than the department’s name. According to the US Census Bureau, the South Carolina’s population has almost doubled since 1950 and the majority of our citizens now live in urban areas. -

Arthropoda: Pycnogonida)
European Journal of Taxonomy 286: 1–33 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2017.286 www.europeanjournaloftaxonomy.eu 2017 · Sabroux R. et al. This work is licensed under a Creative Commons Attribution 3.0 License. DNA Library of Life, research article urn:lsid:zoobank.org:pub:8B9DADD0-415E-4120-A10E-8A3411C1C1A4 Biodiversity and phylogeny of Ammotheidae (Arthropoda: Pycnogonida) Romain SABROUX 1, Laure CORBARI 2, Franz KRAPP 3, Céline BONILLO 4, Stépahnie LE PRIEUR 5 & Alexandre HASSANIN 6,* 1,2,6 UMR 7205, Institut de Systématique, Evolution et Biodiversité, Département Systématique et Evolution, Sorbonne Universités, Muséum national d’Histoire naturelle, 55 rue Buffon, CP 51, 75005 Paris, France. 3 Zoologisches Forschungsmuseum Alexander Koenig, Adenauerallee 160, 53113 Bonn, Germany. 4,5 UMS CNRS 2700, Muséum national d’Histoire naturelle, CP 26, 57 rue Cuvier, 75231 Paris Cedex 05, France. * Corresponding author: [email protected] 1 Email: [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 1 urn:lsid:zoobank.org:author:F48B4ABE-06BD-41B1-B856-A12BE97F9653 2 urn:lsid:zoobank.org:author:9E5EBA7B-C2F2-4F30-9FD5-1A0E49924F13 3 urn:lsid:zoobank.org:author:331AD231-A810-42F9-AF8A-DDC319AA351A 4 urn:lsid:zoobank.org:author:7333D242-0714-41D7-B2DB-6804F8064B13 5 urn:lsid:zoobank.org:author:5C9F4E71-9D73-459F-BABA-7495853B1981 6 urn:lsid:zoobank.org:author:0DCC3E08-B2BA-4A2C-ADA5-1A256F24DAA1 Abstract. The family Ammotheidae is the most diversified group of the class Pycnogonida, with 297 species described in 20 genera. -

PYCNOGONIDS Sea Spiders of California
PYCNOGONIDS Sea Spiders of California Sea spiders are neither spiders nor crustaceans. They are a separate class of the phylum Arthropoda (or a separate sub phylum according to some). They do, however, have considerable gross anatomical similarity to spiders. The following comments on the general anatomy and natural history of sea spiders are drawn in large part from a semi-popular synopsis by King (1974) augmented with observations by others (particularly Bouvier 1923). King's account is recommended to all interested in the ecology or taxonomy of California sea spiders. STRUCTURE most pycnogonids have four pairs of legs although species with five or six pairs are found elsewhere in the world (Fry and Hedgpeth 1969), and may be located in waters offshore California An anterior proboscis, a dorsal ocular tubercle, and a posterior abdomen are found along the midline of the body (Fig. 1). The ocular tubercle may either be absent (as m the aberrant interstitial Rhqnchothorax) or may range from a low nub to a long thin "turret" raised far above the dorsum. It may be followed by one or more anoculate tubercles along the dorsal midline. The abdomen may in some species protrude above the dorsum rather than posteriorly. Three other types of appendages occur: chelifores, palps, and ovigers. Chelifores are short, 1-4 segmented limbs above the proboscis, usually ending in chelae. Posterioventral from these a pair of palps are located in some species Chelifores and palps are not present in all species, and their structure varies with age in some families. The chelifores may be chelate in the young and achelate in the adult (eg. -

Larvae of the Pycnogonids Ammothea Gigantea Gordon, 1932 and Achelia Cuneatis Child, 1999 Described from Archived Specimens
Arthropods, 2012, 1(4):121-128 Article Larvae of the pycnogonids Ammothea gigantea Gordon, 1932 and Achelia cuneatis Child, 1999 described from archived specimens John A. Fornshell, Frank D. Ferrari National Museum of Natural History, Department of invertebrate Zoology, Smithsonian Institution, 4210 Silver Hill Road Suitland, Maryland 20746, USA E-mail: [email protected] Received 30 July 2012; Accepted 2 September 2012; Published online 1 December 2012 IAEES Abstract The larvae of two species of Pycnogonida are described from archived collections. Achelia cuneatis Child, 1999 is an example of a typical protonymphon larva in having three pairs of three segmented appendages, cheliphores, palps and ovigerous appendages. Ammothea gigantea Gordon, 1932 is an example of a lecithotrophic protonymphon larva, having the three pair of appendages plus buds of the first walking legs. Ammothea gigantea is the third species known to have this larval type. The Achelia cuneatis protonymphon larvae is oval, 150 µm long and characterized by a relatively long spinneret spine arising at the distal end of the first segment of each cheliphore. Spines also arise from the distal end of the first segment of the second and third limbs. This typical protonymphon larva of Achelia cuneatis has few yolk granules. The lecithotrophic protonymphon of Ammothea gigantea lacks a spinneret spine on the cheliphore; the larva is oval in shape, but much larger than Achelia cuneatis with a length of 700 µm. Its second limb is much larger than the third limb on the first post-embryonic stage, and the third limb is further reduced to a spine-like structure on the second post-embryonic stage. -

Biodiversity and Phylogeny of Ammotheidae
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: European Journal of Taxonomy Jahr/Year: 2017 Band/Volume: 0286 Autor(en)/Author(s): Sabroux Romain, Corbari Laure, Krapp Franz, Bonillo Celine, Le Prieur Stephanie, Hassanin Alexandre Artikel/Article: Biodiversity and phylogeny of Ammotheidae (Arthropoda: Pycnogonida) 1-33 European Journal of Taxonomy 286: 1–33 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2017.286 www.europeanjournaloftaxonomy.eu 2017 · Sabroux R. et al. This work is licensed under a Creative Commons Attribution 3.0 License. DNA Library of Life, research article urn:lsid:zoobank.org:pub:8B9DADD0-415E-4120-A10E-8A3411C1C1A4 Biodiversity and phylogeny of Ammotheidae (Arthropoda: Pycnogonida) Romain SABROUX 1, Laure CORBARI 2, Franz KRAPP 3, Céline BONILLO 4, Stépahnie LE PRIEUR 5 & Alexandre HASSANIN 6,* 1,2,6 UMR 7205, Institut de Systématique, Evolution et Biodiversité, Département Systématique et Evolution, Sorbonne Universités, Muséum national d’Histoire naturelle, 55 rue Buffon, CP 51, 75005 Paris, France. 3 Zoologisches Forschungsmuseum Alexander Koenig, Adenauerallee 160, 53113 Bonn, Germany. 4,5 UMS CNRS 2700, Muséum national d’Histoire naturelle, CP 26, 57 rue Cuvier, 75231 Paris Cedex 05, France. * Corresponding author: [email protected] 1 Email: [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 1 urn:lsid:zoobank.org:author:F48B4ABE-06BD-41B1-B856-A12BE97F9653 2 urn:lsid:zoobank.org:author:9E5EBA7B-C2F2-4F30-9FD5-1A0E49924F13 3 urn:lsid:zoobank.org:author:331AD231-A810-42F9-AF8A-DDC319AA351A 4 urn:lsid:zoobank.org:author:7333D242-0714-41D7-B2DB-6804F8064B13 5 urn:lsid:zoobank.org:author:5C9F4E71-9D73-459F-BABA-7495853B1981 6 urn:lsid:zoobank.org:author:0DCC3E08-B2BA-4A2C-ADA5-1A256F24DAA1 Abstract. -

Visual System of Basal Chelicerata
Visual system of basal Chelicerata Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.) der Fakultät für Biologie der Ludwig‐Maximilians‐Universität München Vorgelegt von Dipl.‐Biol. Tobias Lehmann München, 2014 Cover: left, Achelia langi (Dohrn, 1881); right, Euscorpius italicus (Herbst, 1800); photos by the author. 1. Gutachter: Prof. Dr. Roland R. Melzer 2. Gutachter: Prof. Dr. Gerhard Haszprunar Tag der mündlichen Prüfung: 22. Oktober 2014 i ii Erklärung Diese Dissertation wurde im Sinne von § 12 der Promotionsordnung von Prof. Dr. Roland R. Melzer betreut. Ich erkläre hiermit, dass die Dissertation nicht einer anderen Prüfungskommission vorgelegt worden ist und dass ich mich nicht anderweitig einer Doktorprüfung ohne Erfolg unterzogen habe. ____________________________ ____________________________ Ort, Datum Dipl.‐Biol. Tobias Lehmann Eidesstattliche Erklärung Ich versichere hiermit an Eides statt, dass die vorgelegte Dissertation von mir selbständig und ohne unerlaubte Hilfe angefertigt ist. ____________________________ ____________________________ Ort, Datum Dipl.‐Biol. Tobias Lehmann iii List of publications Paper I Lehmann T, Heß M & Melzer RR (2012). Wiring a Periscope – Ocelli, Retinula Axons, Visual Neuropils and the Ancestrality of Sea Spiders. PLoS ONE 7 (1): 30474. doi:10.1371/journal. pone.0030474. Paper II Lehmann T & Melzer RR (2013). Looking like Limulus? – Retinula axons and visual neuropils of the median and lateral eyes of scorpions. Frontiers in Zoology 10 (1): 40. doi:10.1186/1742‐9994‐10‐40. Paper III (under review) Lehmann T, Heß M, Wanner G & Melzer RR (2014). Dissecting an ancestral neuron network – FIB‐SEM based 3D‐reconstruction of the visual neuropils in the sea spider Achelia langi (Dohrn, 1881) (Pycnogonida). BMC Biology: under review. -

Body Size in Antarctic and Temperate Sea Spiders: the Role Of
BODY SIZE IN ANTARCTIC AND TEMPERATE SEA SPIDERS: THE ROLE OF TEMPERATURE AND OXYGEN A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI‘I AT MĀNOA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY (Ecology, Evolution and Conservation Biology) August 2019 By Caitlin Mariko Uʻilani Shishido Dissertation Committee: Amy Moran, Chairperson Robert Toonen H. Arthur Woods Amber Wright Celia Smith Keywords: Pycnogonid, temperature, body size, climate change Acknowledgments I would like to thank the staff and directors of labs where I have done this dissertation work including the UH Mānoa Biology Department, Kewalo Marine Laboratory, Friday Harbor Laboratories, Oregon Institute of Marine Biology, and McMurdo Station, Antarctica for field and technical support. Thank you to the McMurdo dive supervisors Rob Robbins and Steve Rupp for SCUBA support and for sharing their infinite knowledge of the underwater world of Antarctica. I would also thank Peter Marko, Michael Wallstrom, Floyd Reed, Shannon Wells-Moran, Sachie Etherington, and the BIOL 375L class from Fall 2016 at the University of Hawai‘i at Mānoa for their contributions to the barcoding effort. Funding for this work was provided by NSF grant PLR-1341476 to Amy Moran and PLR-1341485 to Art Woods and Bret Tobalske. A very special thank you to my collaborators Steve Lane, Bret Tobalske, Art Woods for endless discussions on the obscure topic of pycnogonids, for keeping me awake during pisten bully driving, and for an unforgettable experience in Antarctica. Thank you also to Tim Dwyer for additional SCUBA support in Antarctica and permission to use his amazing photographs. -

Jahresbericht 2012 Der Generaldirektion Der Staatlichen Naturwissenschaftlichen Sammlungen Bayerns Herausgegeben Von: Prof
Jahresbericht 2012 der Generaldirektion der Staatlichen Naturwissenschaftlichen Sammlungen Bayerns Herausgegeben von: Prof. Dr. Gerhard Haszprunar, Generaldirektor Generaldirektion der Staatlichen Naturwissenschaftlichen Sammlungen Bayerns (SNSB) Menzinger Straße 71, 80638 München München September 2013 Zusammenstellung und Endredaktion: Dr. Eva Maria Natzer (Generaldirektion) Unterstützung durch: Maria-Luise Kaim (Generaldirektion) Iris Krumböck (Generaldirektion) Katja Henßel (Generaldirektion) Druck: Digitaldruckzentrum, Amalienstrasse, München Inhaltsverzeichnis Bericht des Generaldirektors ...................................................................................................4 Wissenschaftliche Publikationen ................................................................................................6 Drittmittelübersicht ...................................................................................................................42 Organigramm ............................................................................................................................55 Generaldirektion .....................................................................................................................56 Personalvertretung ....................................................................................................................58 Museen Museum Mensch und Natur (MMN) ........................................................................................59 Museum Reich der Kristalle (MRK) ........................................................................................67 -

Larval Types, Courtship and Mating Behaviors, and the Costs
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Oregon Scholars' Bank LARVAL TYPES, COURTSHIP AND MATING BEHAVIORS, AND THE COSTS ASSOCIATED WITH EXCLUSIVE MALE PARENTAL CARE IN THE SEA SPIDER ACHELIA SIMPLISSIMA (PYCNOGONIDA) by ZAIR P. BURRIS A THESIS Presented to the Department ofBiology and the Graduate School ofthe University ofOregon in partial fulfillment ofthe requirements for the degree of Master of Science September 2010 11 "Larval Types, Courtship and Mating Behaviors, and the Costs Associated with Exclusive Male Parental Care in the Sea Spider Achelia simplissima (Pycnogonida)," a thesis prepared by Zair P. Burris in partial fulfillment ofthe requirements for the Master ofScience degree in the Department ofBiology. This thesis has been approved and accepted by: Date /2~ ~,------2_()_I(-)--- Committee in Charge: Alan Shanks, Chair Svetlana Maslakova Craig Young Accepted by: Dean ofthe Graduate School 111 An Abstract ofthe Thesis of Zair P. Burris for the degree of Master of Science in the Department of Biology to be taken September 2010 Title: LARVAL TYPES, COURTSHIP AND MATING BEHAVIORS, AND THE COSTS ASSOCIATED WITH EXCLUSIVE MALE PARENTAL CARE IN THE ~~.IA SIMPLISSlMA (PYCNOGONIDA) - Alan Shanks In all species ofpycnogonids (sea spiders) males care exclusively for the offspring, making this group essential for studies on sex roles, sexual selection, and the evolution ofparental investment. Unfortunately, little is known about pycnogonid mating patterns, larval development, or the costs associated with parental care. The mating habits ofboth male and female Achelia simplissima were studied experimentally and reveal that both sexes routinely mate multiple times and have multiple mates. -

Morphological and Molecular Phylogenetic Analysis of the Sea Spiders (Arthropoda, Pycnogonida) and Taxonomic Study of Tropical Australian Forms
ResearchOnline@JCU This file is part of the following reference: Arango, Claudia Patricia (2002) Morphological and molecular phylogenetic analysis of the sea spiders (Arthropoda, Pycnogonida) and taxonomic study of tropical Australian forms. PhD thesis, James Cook University. Access to this file is available from: http://eprints.jcu.edu.au/24091/ The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owner of any third party copyright material included in this document. If you believe that this is not the case, please contact [email protected] and quote http://eprints.jcu.edu.au/24091/ Morphological and Molecular Phylogenetic Analysis of the Sea Spiders (Arthropoda, Pycnogonida) and Taxonomic Study of Tropical Australian forms Thesis submitted by Claudia Patricia Arango BSc in February 2002 For the degree of Doctor of Philosophy School of Tropical Biology & School of Marine Biology and Aquaculture James Cook University Statement of access I, the undersigned, the author of this thesis, understand that James Cook University will make it available for use within the University library and, by microfilm or other means, allow access to users in other approved libraries. All users consulting this thesis will have to sign the following statement: In consulting this thesis I agree not to copy or closely paraphrase it in whole or in part without the written consent of the author; and to make proper public written acknowledgment for any assistance that I have obtained from it. Beyond this, I do not wish to place any restriction on access to this thesis. Ws el 2002 Date ii ACKNOWLEDGEMENTS First of all I thank my family for their immense love and support.