Soil Organisms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

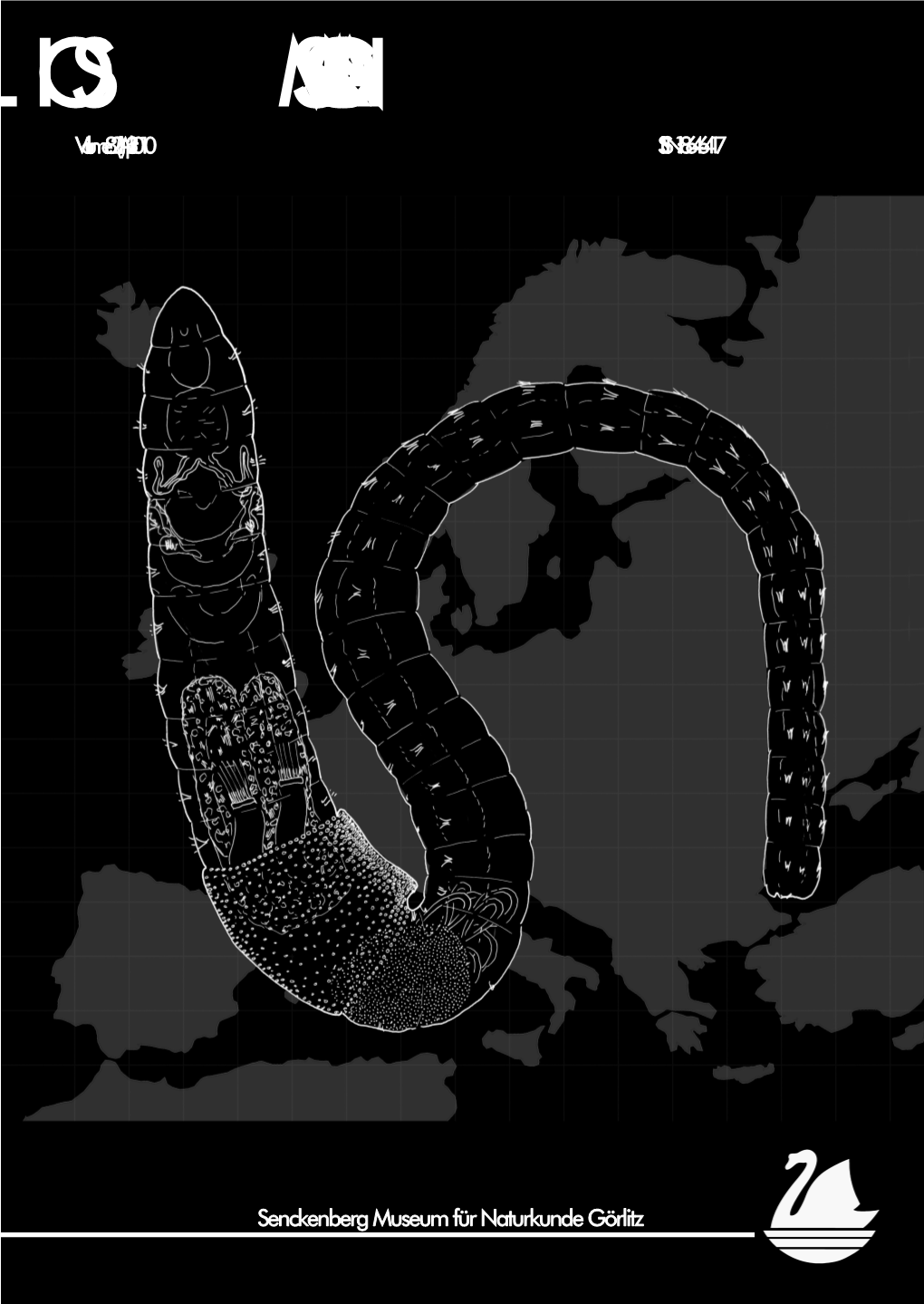
'Cejkaian Tubules' in the Posterior Midgut of Terrestrial Enchytraeidae (Oligochaeta)
85 (2) · August 2013 pp. 113–122 ‘Cejkaian tubules’ in the posterior midgut of terrestrial Enchytraeidae (Oligochaeta) Rüdiger M. Schmelz1,2,* and Rut Collado2 1 ECT Oekotoxikologie GmbH, Böttgerstrasse 2–14, 65439 Flörsheim am Main, Germany 2 Universidad de A Coruña, Fac. Ciencias, Dep. Biología Animal, Biol. Vegetal, y Ecología, Rua da Fraga 10, 15008 A Coruña, Spain * Corresponding author, e-mail: [email protected] Received 29 May 2013 | Accepted 2 July 2013 Published online at www.soil-organisms.de 1 August 2013 | Printed version 15 August 2013 Abstract More than one hundred years ago, Bohumil Čejka described peculiar elongate tubules in the posterior region of the intestine of Hepatogaster birulae, a new terrestrial enchytraeid species collected in North-East Siberia. The tubules have no cilia but a proper epithelium and they run parallel to the longitudinal axis of the intestine over several segments, inside the intestinal epithelium but in close contact with the blood sinus. The tubules end blindly anteriorly and with a porus to the intestinal lumen posteriorly. The number of tubules increases from posterior to anterior due to bifurcations, and their diameter decreases. Čejka hypothesized that these tubules are glands that provide secretions for the final process of digestion or that aid in the egestion of faeces. He found them only in one species, Hepatogaster birulae, which was later synonymized with Henlea ochracea. In recent years we screened a large number of terrestrial enchytraeids in vivo and found these peculiar tubules in two further species of Henlea, in one species of Oconnorella and in thirteen species of Fridericia. -

DNA-Based Environmental Monitoring for the Invasive Myxozoan Parasite, Myxobolus Cerebralis, in Alberta, Canada
! ! ! ! "#$%&'()*!+,-./0,1),2'3!40,.20/.,5!60/!27)!!8,-'(.-)!49:0;0',!<'/'(.2)=!!"#$%$&'() *+,+%,-&.(=!.,!$3>)/2'=!?','*'! ! >9! ! "',.)33)!+/.,!&'//9! ! ! ! ! ! ! ! ! $!27)(.(!(@>1.22)*!.,!A'/2.'[email protected]),2!06!27)!/)B@./)1),2(!60/!27)!*)5/))!06! ! ! 4'(2)/!06!CD.),D)! ! .,! ! +,-./0,1),2'3!E)'327!CD.),D)(! ! ! ! ! ! CD7003!06!<@>3.D!E)'327! F,.-)/(.29!06!$3>)/2'! ! ! ! ! ! ! ! ! ! ! ! G!"',.)33)!+/.,!&'//9=!HIHI! !! ! ! ! ! ! !"#$%&'$( ! J7./3.,5!*.()'()!.(!'!*.()'()!06!6.(7!D'@()*!>9!',!.,-'(.-)!19:0(A0/)',!A'/'(.2)=! !"#$%$&'()*+,+%,-&.(K!82!L'(!6./(2!*)2)D2)*!.,!?','*'!.,!M07,(0,!N'O)!.,!&',66!#'2.0,'3!<'/O=! $3>)/2'=!.,!$@5@(2!HIPQ=!',*!3.223)!.(!O,0L,!'>0@2!27)!2/',(1.((.0,!06!27.(!A'/'(.2)!.,!?','*'K! ?@//),2!2)(2.,5!60D@()(!0,!27)!*)2)D2.0,!06!!/)*+,+%,-&.(!.,!6.(7!2.((@)(=!/)B@./.,5!3)27'3!2)(2.,5!06! >027!.,6)D2)*!',*!,0,%.,6)D2)*!6.(7K!E0L)-)/=!27)!A'/'(.2)!7'(!'!*)6.,.2.-)!70(2=!27)!03.50D7')2)! L0/1!0'%.1+#)2'%.1+#!',*!2L0!),-./0,1),2'3!(2'5)(!60@,*!.,!L'2)/!',*!()*.1),2!27'2!D/)'2)! 027)/!'-),@)(!60/!*)2)D2.0,K!J)!A/0A0()!27'2!@(.,5!27)!A'/'(.2)!(2'5)(!60@,*!.,!L'2)/!',*! ()*.1),2!',*!27)!'32)/,'2)!L0/1!70(2=!0'%.1+#)2'%.1+#3!'/)!'!/)'(0,'>3)!D01A3)1),2!20!6.(7! ('1A3.,5!',*!L.33!>)!)(A)D.'339!@()6@3!60/!('1A3.,5!.,!'/)'(!L7)/)!6.(7!D033)D2.0,!.(!D7'33),5.,5! 0/!A/07.>.2.-)!*@)!20!-@3,)/'>.3.29!06!27)!6.(7!A0A@3'2.0,(K!8,!'**.2.0,=!0/)2'%.1+#!(@(D)A2.>.3.29!20! !/)*+,+%,-&.(!.(!,02!D0,(.(2),2!'D/0((!27)!(A)D.)(=!L.27!):A)/.1),2(!(70L.,5!(01)!'/)!/)6/'D20/9K! ?7'/'D2)/.;'2.0,!06!27)()!L0/1!A0A@3'2.0,(!L.33!7)3A!2'/5)2!6@2@/)!10,.20/.,5!',*!D0,2/03! -

Testing Species Hypotheses for Fridericia Magna, an Enchytraeid Worm (Annelida: Clitellata) with Great Mitochondrial Variation
Martinsson et al. BMC Evolutionary Biology (2020) 20:116 https://doi.org/10.1186/s12862-020-01678-5 RESEARCH ARTICLE Open Access Testing species hypotheses for Fridericia magna, an enchytraeid worm (Annelida: Clitellata) with great mitochondrial variation Svante Martinsson* , Mårten Klinth and Christer Erséus Abstract Background: Deep mitochondrial divergences were observed in Scandinavian populations of the terrestrial to semi-aquatic annelid Fridericia magna (Clitellata: Enchytraeidae). This raised the need for testing whether the taxon is a single species or a complex of cryptic species. Results: A total of 62 specimens from 38 localities were included in the study, 44 of which were used for species delimitation. First, the 44 specimens were divided into clusters using ABGD (Automatic Barcode Gap Discovery) on two datasets, consisting of sequences of the mitochondrial markers COI and 16S. For each dataset, the worms were divided into six not completely congruent clusters. When they were combined, a maximum of seven clusters, or species hypotheses, were obtained, and the seven clusters were used as input in downstream analyses. We tested these hypotheses by constructing haplowebs for two nuclear markers, H3 and ITS, and in both haplowebs the specimens appeared as a single species. Multi-locus species delimitation analyses performed with the Bayesian BPP program also mainly supported a single species. Furthermore, no apparent morphological differences were found between the clusters. Two of the clusters were partially separated from each other and the other clusters, but not strongly enough to consider them as separate species. All 62 specimens were used to visualise the Scandinavian distribution, of the species, and to compare with published COI data from other Fridericia species. -

Redalyc.CONTINENTAL BIODIVERSITY of SOUTH
Acta Zoológica Mexicana (nueva serie) ISSN: 0065-1737 [email protected] Instituto de Ecología, A.C. México Christoffersen, Martin Lindsey CONTINENTAL BIODIVERSITY OF SOUTH AMERICAN OLIGOCHAETES: THE IMPORTANCE OF INVENTORIES Acta Zoológica Mexicana (nueva serie), núm. 2, 2010, pp. 35-46 Instituto de Ecología, A.C. Xalapa, México Available in: http://www.redalyc.org/articulo.oa?id=57515556003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative ISSN 0065-1737 Acta ZoológicaActa Zoológica Mexicana Mexicana (n.s.) Número (n.s.) Número Especial Especial 2: 35-46 2 (2010) CONTINENTAL BIODIVERSITY OF SOUTH AMERICAN OLIGOCHAETES: THE IMPORTANCE OF INVENTORIES Martin Lindsey CHRISTOFFERSEN Universidade Federal da Paraíba, Departamento de Sistemática e Ecologia, 58.059-900, João Pessoa, Paraíba, Brasil. E-mail: [email protected] Christoffersen, M. L. 2010. Continental biodiversity of South American oligochaetes: The importance of inventories. Acta Zoológica Mexicana (n.s.), Número Especial 2: 35-46. ABSTRACT. A reevaluation of South American oligochaetes produced 871 known species. Megadrile earthworms have rates of endemism around 90% in South America, while Enchytraeidae have less than 75% endemism, and aquatic oligochaetes have less than 40% endemic taxa in South America. Glossoscolecid species number 429 species in South America alone, a full two-thirds of the known megadrile earthworms. More than half of the South American taxa of Oligochaeta (424) occur in Brazil, being followed by Argentina (208 taxa), Ecuador (163 taxa), and Colombia (142 taxa). -

Descripción De Nuevas Especies Animales De La Península Ibérica E Islas Baleares (1978-1994): Tendencias Taxonómicas Y Listado Sistemático
Graellsia, 53: 111-175 (1997) DESCRIPCIÓN DE NUEVAS ESPECIES ANIMALES DE LA PENÍNSULA IBÉRICA E ISLAS BALEARES (1978-1994): TENDENCIAS TAXONÓMICAS Y LISTADO SISTEMÁTICO M. Esteban (*) y B. Sanchiz (*) RESUMEN Durante el periodo 1978-1994 se han descrito cerca de 2.000 especies animales nue- vas para la ciencia en territorio ibérico-balear. Se presenta como apéndice un listado completo de las especies (1978-1993), ordenadas taxonómicamente, así como de sus referencias bibliográficas. Como tendencias generales en este proceso de inventario de la biodiversidad se aprecia un incremento moderado y sostenido en el número de taxones descritos, junto a una cada vez mayor contribución de los autores españoles. Es cada vez mayor el número de especies publicadas en revistas que aparecen en el Science Citation Index, así como el uso del idioma inglés. La mayoría de los phyla, clases u órdenes mues- tran gran variación en la cantidad de especies descritas cada año, dado el pequeño núme- ro absoluto de publicaciones. Los insectos son claramente el colectivo más estudiado, pero se aprecia una disminución en su importancia relativa, asociada al incremento de estudios en grupos poco conocidos como los nematodos. Palabras clave: Biodiversidad; Taxonomía; Península Ibérica; España; Portugal; Baleares. ABSTRACT Description of new animal species from the Iberian Peninsula and Balearic Islands (1978-1994): Taxonomic trends and systematic list During the period 1978-1994 about 2.000 new animal species have been described in the Iberian Peninsula and the Balearic Islands. A complete list of these new species for 1978-1993, taxonomically arranged, and their bibliographic references is given in an appendix. -

Colecciones De Invertebrados Del Museo Nacional De Ciencias Naturales (Csic)
HISTORIA Y PRESENTE DE LAS COLECCIONES DE INVERTEBRADOS DEL MUSEO NACIONAL DE CIENCIAS NATURALES (CSIC) Miguel Villena Sánchez-Valero Conservador de las colecciones de Invertebrados 1 .- RESEÑA HISTÓRICA Para encontrar el origen de la Colección de Invertebrados de Museo Nacional de Ciencias Naturales de Madrid tenemos que remontarnos al último tercio del siglo XVIII, cuando, tras numerosas gestiones y diversos intentos, una Real Orden de Carlos III, promulgada el 17 de octubre de 1771, crea el Real Gabinete de Historia Natural y pone punto final a las enormes carencias que, en ese sentido, tenía España respecto a otros países europeos. En estos momentos iniciales, y hasta que se inaugure de forma definitiva el 4 de noviembre de 1776, las colecciones custodiadas en este establecimiento tendrán como base el excelente Gabinete de Historia Natural formado en París por el sabio ilustrado Pedro Franco Dávila, quien, gracias a sus desvelos en la formación del Gabinete y, sobre todo, gracias a los conocimientos en Historia Natural, adquiridos en una estancia de casi 26 años en París, en contacto con los científicos más reputados del momento, será nombrado Primer Director del establecimiento. Con este nombramiento Don Pedro recibió un triple encargo: que se coloquen en Madrid en debida forma las preciosidades actuales del Gabinete, y las demás con que el Rey providenciará enriquecerle, que se verifique la instrucción pública y, sobre todo, el encargo especial de que le tenga a su cuidado y procure difundir el gusto y nociones de tan importante materia.1 A partir de ese momento el interés principal del Gabinete recién creado y de sus dirigentes será el incremento de las colecciones con ese, cuando menos, triple objetivo que tiene que tener todo Museo2 que se precie de tal, es decir: • Conservar, catalogar, restaurar y exhibir de forma ordenada sus colecciones. -

Two Species of Fridericia Mich., 1889 (Oligochaeta, Enchytraeidae) from Brazil
Bolm. Zool., Univ. S. Paulo 1:239-256, 1976 Two species of Fridericia Mich., 1889 (Oligochaeta, Enchytraeidae) from Brazil. MARTIN LINDSEY CHRISTOFFERSEN Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo SUMMARY Two species of enchytraeid worms common in the State of São Paulo, Fridericia bulbosa (Rosa, 1887) and F bollonsi Benham, 1914, were studied regarding their anatomy and taxonomy. Three varieties of F. bulbosa were recognized which, in a general manner, present the following differences: variations in body size, dif ference in number of body segments and setae, dissimilar disposition of clitellar and chylus cells, variety in shape of spermathecae, peptonephridia and septal glands, different points of origin of ectal ducts on nephridia and of dorsal vessel and, finally, difference in size of seminal funnels and of penial bulbs. RESUMO Foram estudadas anatômica e sistematicamente Fridericia bulbosa (Rosa, 1887) e F. bollonsi Benham, 1914, comuns no Estado de São Paulo. De F. bulbosa foram reconhecidas três variedades que, de um modo geral, diferem pelo tamanho, número de segmentos e de cerdas, disposição das células clitelares e quilíferas, forma das espermatecas, dos peptonefrídios e das glândulas septais, pela origem do duto excretor nos nefrídios e do vaso dorsal, assim como pelo tamanho dos funis seminais e dos bulbos peniais. Being interested in the Oligochaeta, Enchytraeidae, I studied the anthropochorous species, which must be very plastic, anatomically and/or physiologically, to adapt themselves to the new biotopes into which they are introduced by man. Only when the intraspecific differences in the various geographical regions are compiled, will it be possible to establish with certainty the degree of variability of these peregrine species and consequently their synonymy. -

Annelida, Clitellata, Enchytraeidae)
Organisms Diversity & Evolution (2018) 18:291–312 https://doi.org/10.1007/s13127-018-0374-6 ORIGINAL ARTICLE Two new bioluminescent Henlea from Siberia and lack of molecular support for Hepatogaster (Annelida, Clitellata, Enchytraeidae) Emilia Rota1 & Svante Martinsson2 & Christer Erséus2 Received: 25 February 2018 /Accepted: 30 July 2018 /Published online: 30 August 2018 # Gesellschaft für Biologische Systematik 2018 Abstract Two bioluminescent enchytraeids, Henlea petushkovi sp. n. and Henlea rodionovae sp. n., are described from the Krasnoyarsk and Irkutsk regions in Eastern Siberia. These large potworms exhibit the typical light-production pattern reported repeatedly in the genus and recently elucidated by Russian researchers in its main biophysical and biochemical aspects. Morphology and DNA indicate that the two species are very closely related, but clearly divergent in the strength of the body wall (thick and opaque in H. petushkovi), structure of the prostomium (in H. rodionovae unprecedentedly wrinkled and mobile), brain shape (almost equilat- eral in H. petushkovi), size of coelomocytes (60–85 μminH. petushkovi) and structure of intestinal diverticula (tulip-shaped in H. petushkovi, apple-shaped in H. rodionovae). Limited hybridization seems to occur between them, supported by a single case of conflict between COI and morphology, and a few intermediate morphotypes were noted in greenhouse populations. The new species are phylogenetically distant from all known congeners so far DNA-barcoded, even those that, like them, respond to the diagnosis of the putative subgenus Hepatogaster Čejka, 1910 (multitubular gut diverticula in VIII, indented brain, dorsal blood vessel from IX, prominent spermathecal glands, and nephridia from 5/6). In fact, our phylogenetic analyses dismiss Hepatogaster as an artificial (polyphyletic) taxon. -

Biological Assessment of the Patapsco River Tributary Watersheds, Howard County, Maryland
Biological Assessment of the Patapsco River Tributary Watersheds, Howard County, Maryland Spring 2003 Index Period and Summary of Round One County- Wide Assessment Patuxtent River April, 2005 Final Report UT to Patuxtent River Biological Assessment of the Patapsco River Tributary Watersheds, Howard County, Maryland Spring 2003 Index Period and Summary of Round One County-wide Assessment Prepared for: Howard County, Maryland Department of Public Works Stormwater Management Division 6751 Columbia Gateway Dr., Ste. 514 Columbia, MD 21046-3143 Prepared by: Tetra Tech, Inc. 400 Red Brook Blvd., Ste. 200 Owings Mills, MD 21117 Acknowledgement The principal authors of this report are Kristen L. Pavlik and James B. Stribling, both of Tetra Tech. They were also assisted by Erik W. Leppo. This document reports results from three of the six subwatersheds sampled during the Spring Index Period of the third year of biomonitoring by the Howard County Stormwater Management Division. Fieldwork was conducted by Tetra Tech staff including Kristen Pavlik, Colin Hill, David Bressler, Jennifer Pitt, and Amanda Richardson. All laboratory sample processing was conducted by Carolina Gallardo, Shabaan Fundi, Curt Kleinsorg, Chad Bogues, Joey Rizzo, Elizabeth Yarborough, Jessica Garrish, Chris Hines, and Sara Waddell. Taxonomic identification was completed by Dr. R. Deedee Kathman and Todd Askegaard; Aquatic Resources Center (ARC). Hunt Loftin, Linda Shook, and Brenda Decker (Tetra Tech) assisted with budget tracking and clerical support. This work was completed under the Howard County Purchase Order L 5305 to Tetra Tech, Inc. The enthusiasm and interest of the staff in the Stormwater Management Division, including Howard Saltzman and Angela Morales is acknowledged and appreciated. -

Nabs 2004 Final
CURRENT AND SELECTED BIBLIOGRAPHIES ON BENTHIC BIOLOGY 2004 Published August, 2005 North American Benthological Society 2 FOREWORD “Current and Selected Bibliographies on Benthic Biology” is published annu- ally for the members of the North American Benthological Society, and summarizes titles of articles published during the previous year. Pertinent titles prior to that year are also included if they have not been cited in previous reviews. I wish to thank each of the members of the NABS Literature Review Committee for providing bibliographic information for the 2004 NABS BIBLIOGRAPHY. I would also like to thank Elizabeth Wohlgemuth, INHS Librarian, and library assis- tants Anna FitzSimmons, Jessica Beverly, and Elizabeth Day, for their assistance in putting the 2004 bibliography together. Membership in the North American Benthological Society may be obtained by contacting Ms. Lucinda B. Johnson, Natural Resources Research Institute, Uni- versity of Minnesota, 5013 Miller Trunk Highway, Duluth, MN 55811. Phone: 218/720-4251. email:[email protected]. Dr. Donald W. Webb, Editor NABS Bibliography Illinois Natural History Survey Center for Biodiversity 607 East Peabody Drive Champaign, IL 61820 217/333-6846 e-mail: [email protected] 3 CONTENTS PERIPHYTON: Christine L. Weilhoefer, Environmental Science and Resources, Portland State University, Portland, O97207.................................5 ANNELIDA (Oligochaeta, etc.): Mark J. Wetzel, Center for Biodiversity, Illinois Natural History Survey, 607 East Peabody Drive, Champaign, IL 61820.................................................................................................................6 ANNELIDA (Hirudinea): Donald J. Klemm, Ecosystems Research Branch (MS-642), Ecological Exposure Research Division, National Exposure Re- search Laboratory, Office of Research & Development, U.S. Environmental Protection Agency, 26 W. Martin Luther King Dr., Cincinnati, OH 45268- 0001 and William E. -

Diversity Patterns in Neotropical Collembola: Elevational Gradients
Dec 2015 Vol.6, Issue 4 Diversity Patterns in Neotropical Collembola: Investigating the Significance of Elevational Gradients News Applications Updates from Estimating Symposia Held Coextinction Around the Rates through World DNA Barcoding News Briefs The Slovak National Museum- Natural History Museum obtained financial support of 1.7 M € from the EU European Regional Development Fund for Welcome to our December 2015 issue. building a DNA lab and other infrastructure to barcode the Another eventful year has passed with the 6th International Barcode flora and fauna of Slovakia in of Life conference as a fantastic highlight. 600 researchers from 50 2016 – 2023. With the added nations, over 200 talks, more than 100 posters - far more than our little capacity, the museum plans newsletter can ever convey even in a year with 4 jam-packed issues. to barcode 1000 species in the coming years. Nevertheless, we are looking back at another successful year, and we will try to keep the momentum going that the conference started. The German Barcode of Life Network (GBOL) was awarded This issue contains more prize winners from the conference and a lot a further 6.3 M € by the German of good news with respect to funding and national initiatives. Federal Ministry of Education and Research to extend the German We wish you a happy holiday season and a healthy and prosperous barcode reference library to New Year. contain all common and frequent species, as well as important agricultural pests, invasive, Dirk Steinke health-relevant, Red List, FFH Editor-in-chief (Flora Fauna Habitat Directive), indicator and specific application- relevant species, and to develop Table of Contents DNA barcoding applications. -

Freshwater Oligochaetes (Oligochaeta, Clitellata, Annelida) of North Pribaikalye (East Siberia, Russia)
I. A. KAYGORODOVA, P. F. M. VERDONSCHOT, L. S. KRAVTSOVA Turk J Zool 2012; 36(1): 47-58 © TÜBİTAK Research Article doi:10.3906/zoo-1003-140 Freshwater oligochaetes (Oligochaeta, Clitellata, Annelida) of North Pribaikalye (East Siberia, Russia) Irina A. KAYGORODOVA1,*, Piet F. M. VERDONSCHOT2, Lyubov S. KRAVTSOVA1 1Limnological Institute, Siberian Branch of Russian Academy of Sciences, Ulan-Batorskaya, 3, 664033 Irkutsk - RUSSIA 2Freshwater Ecology Team, Alterra, Centre for Ecosystem Studies, P.O. Box 47, 6700 AA Wageningen - THE NETHERLANDS Received: 24.03.2010 Abstract: Th e oligochaete fauna of freshwater reservoirs and rivers in North Pribaikalye was investigated for the fi rst time. In total, 38 oligochaete species were collected. Of these, 16 species belong to the subfamily Naidinae, 6 to Pristininae, and 10 to the 3 subfamilies of Tubifi cidae. In addition, 5 species belonging to Enchytraeidae and 1 widespread species belonging to the family Lumbriculidae, Lumbriculus variegatus, were collected. Key words: Oligochaeta, fauna, North Pribaikalye Introduction Th e rivers and lakes start freezing between 25 Th e area of Pribaikalye is situated in East Siberia October and 30 October. Th e average duration of (the Asian part of Russia) and includes Lake Baikal. the ice cover is about 200 days. In late May, all water Th e area is dissected by a well-developed river and reservoirs and rivers of North Pribaikalye are usually a regularly divided river network with a density in free of ice cover. the north exceeding 0.5 km km−2 (Bachurin, 1980). Th e temperature of the littoral zone of the lakes Small- and medium-sized rivers fl ow down from the can exceed 20 °C in summer and fl uctuates between slopes of the Verkhne-Angarsk Ridge.