Trait and State Anxiety Are Mapped Differently in the Human Brain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thinking About Actions: the Neural Substrates of Person Knowledge
Person Knowledge 1 Running Head: Person Knowledge Thinking About Actions: The Neural Substrates of Person Knowledge Malia F. Mason, Jane F. Banfield, and C. Neil Macrae Dartmouth College Address Correspondence To: Malia Mason Columbia Business School 720 Uris Hall New York, NY 10027 Email: [email protected] Phone: 212.854.1070 Person Knowledge 2 Abstract Despite an extensive literature on the neural substrates of semantic knowledge, how person-related information is represented in the brain has yet to be elucidated. Accordingly, in the present study we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates of person knowledge. Focusing on the neural substrates of action knowledge, participants reported whether or not a common set of behaviors could be performed by people or dogs. While dogs and people are capable of performing many of the same actions (e.g., run, sit, bite), we surmised that the representation of this knowledge would be associated with distinct patterns of neural activity. Specifically, person judgments were expected to activate cortical areas associated with theory of mind (ToM) reasoning. The results supported this prediction. Whereas action-related judgments about dogs were associated with activity in various regions, including the occipital and parahippocampal gyri; identical judgments about people yielded activity in areas of prefrontal cortex, notably the right middle and medial frontal gyri. These findings suggest that person knowledge may be functionally dissociable from comparable information about other animals, with action-related judgments about people recruiting neural activity that is indicative of ToM reasoning. Key Words: Action Knowledge; fMRI; Social Cognition; Theory of Mind; Mentalizing. -

Anatomy of the Temporal Lobe
Hindawi Publishing Corporation Epilepsy Research and Treatment Volume 2012, Article ID 176157, 12 pages doi:10.1155/2012/176157 Review Article AnatomyoftheTemporalLobe J. A. Kiernan Department of Anatomy and Cell Biology, The University of Western Ontario, London, ON, Canada N6A 5C1 Correspondence should be addressed to J. A. Kiernan, [email protected] Received 6 October 2011; Accepted 3 December 2011 Academic Editor: Seyed M. Mirsattari Copyright © 2012 J. A. Kiernan. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Only primates have temporal lobes, which are largest in man, accommodating 17% of the cerebral cortex and including areas with auditory, olfactory, vestibular, visual and linguistic functions. The hippocampal formation, on the medial side of the lobe, includes the parahippocampal gyrus, subiculum, hippocampus, dentate gyrus, and associated white matter, notably the fimbria, whose fibres continue into the fornix. The hippocampus is an inrolled gyrus that bulges into the temporal horn of the lateral ventricle. Association fibres connect all parts of the cerebral cortex with the parahippocampal gyrus and subiculum, which in turn project to the dentate gyrus. The largest efferent projection of the subiculum and hippocampus is through the fornix to the hypothalamus. The choroid fissure, alongside the fimbria, separates the temporal lobe from the optic tract, hypothalamus and midbrain. The amygdala comprises several nuclei on the medial aspect of the temporal lobe, mostly anterior the hippocampus and indenting the tip of the temporal horn. The amygdala receives input from the olfactory bulb and from association cortex for other modalities of sensation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

Interference Resolution: Insights from a Meta-Analysis of Neuroimaging Tasks
Cognitive, Affective, & Behavioral Neuroscience 2007, 7 (1), 1-17 Interference resolution: Insights from a meta-analysis of neuroimaging tasks DEREK EVA N NEE University of Michigan, Ann Arbor, Michigan TOR D. WAGER Columbia University, New York, New York AND JOHN JONIDES University of Michigan, Ann Arbor, Michigan A quantitative meta-analysis was performed on 47 neuroimaging studies involving tasks purported to require the resolution of interference. The tasks included the Stroop, flanker, go/no-go, stimulus–response compatibil- ity, Simon, and stop signal tasks. Peak density-based analyses of these combined tasks reveal that the anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal gyrus, posterior parietal cortex, and anterior insula may be important sites for the detection and/or resolution of interference. Individual task analyses reveal differential patterns of activation among the tasks. We propose that the drawing of distinctions among the pro- cessing stages at which interference may be resolved may explain regional activation differences. Our analyses suggest that resolution processes acting upon stimulus encoding, response selection, and response execution may recruit different neural regions. The need to select information among competing al- shows the activations arising just from the Stroop task ternatives is ubiquitous. Oftentimes, successful cognition (Stroop, 1935), and these do not appear any more orderly. depends on the ability to focus resources on goal-relevant Indeed, the variability among the reported peaks across information while filtering out or inhibiting irrelevant infor- all interference resolution tasks corroborates behavioral mation. How selective attention operates and whether and findings that correlations in performance among different how irrelevant information is inhibited or otherwise filtered interference resolution tasks are low (Kramer, Humphrey, out has been a major focus of research since the inception Larish, Logan, & Strayer, 1994; Shilling, Chetwynd, & of experimental psychology. -

Lucid Dreaming and the Prefrontal Cortex Performance III
Lucid Dreaming and the Prefrontal Cortex Performance III Georgia Minkoff and Grace Boyar Briarcliff High School Georgia Minkoff and Grace Boyar Briarcliff High School 2 First, we would like to thank our mentor, Dr. Peter Morgan, and Sarah Hodges from the Yale Medical Research Center for their immense support and guidance throughout our project. We would also like to extend a thank you to our teachers Michael Inglis and Annmarie O’Brien, for supervising us throughout our three years in Science Research and making them such an enlightening experience. Finally, we would like to thank all of the students that participated in our study, whose time and effort was greatly appreciated. Abstract Title: Lucid Dreaming and the Prefrontal Cortex III Name and address: Georgia Minkoff and Grace Boyar School/city/state: Briarcliff High School, Briarcliff Manor, NY 10510 Teacher: Mr. Michael Inglis and Ms. Annmarie O’Brien Mentor: Dr. Peter T. Morgan Scientific discipline: Neurological Science Objectives The purpose of this study is to prove that ventromedial but not the dorsolateral prefrontal cortical functions will prove one’s ability to lucid dream. The precuneus is also tested to distinguish lucid from non-lucid dreamers. Each part of the brain listed above is correlated to cognitive behaviors (i.e.; decision making, risk taking, reaction time, and memory). These functions are observed throughout the study to test their degree of enhancement. Methods Each participant endured a week of intense lucid dreaming induction treatment. They were administered questionnaires, assessments, cognitive computer tasks, and a dream journal. While the questionnaires were only completed once, computer tasks were done at the beginning and end of the study to compare the development of the behaviors tested. -

Revista Brasileira De Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012
Rev Bras Psiquiatr. 2012;34:101-111 Revista Brasileira de Psiquiatria Official Journal of the Brazilian Psychiatric Association Psychiatry Volume 34 • Number 1 • March/2012 REVIEW ARTICLE Neuroimaging in specific phobia disorder: a systematic review of the literature Ila M.P. Linares,1 Clarissa Trzesniak,1 Marcos Hortes N. Chagas,1 Jaime E. C. Hallak,1 Antonio E. Nardi,2 José Alexandre S. Crippa1 ¹ Department of Neuroscience and Behavior of the Ribeirão Preto Medical School, Universidade de São Paulo (FMRP-USP). INCT Translational Medicine (CNPq). São Paulo, Brazil 2 Panic & Respiration Laboratory. Institute of Psychiatry, Universidade Federal do Rio de Janeiro (UFRJ). INCT Translational Medicine (CNPq). Rio de Janeiro, Brazil Received on August 03, 2011; accepted on October 12, 2011 DESCRIPTORS Abstract Neuroimaging; Objective: Specific phobia (SP) is characterized by irrational fear associated with avoidance of Specific Phobia; specific stimuli. In recent years, neuroimaging techniques have been used in an attempt to better Review; understand the neurobiology of anxiety disorders. The objective of this study was to perform a Anxiety Disorder; systematic review of articles that used neuroimaging techniques to study SP. Method: A literature Phobia. search was conducted through electronic databases, using the keywords: imaging, neuroimaging, PET, spectroscopy, functional magnetic resonance, structural magnetic resonance, SPECT, MRI, DTI, and tractography, combined with simple phobia and specific phobia. One-hundred fifteen articles were found, of which 38 were selected for the present review. From these, 24 used fMRI, 11 used PET, 1 used SPECT, 2 used structural MRI, and none used spectroscopy. Result: The search showed that studies in this area were published recently and that the neuroanatomic substrate of SP has not yet been consolidated. -

Acetylcholinesterase Staining in Human Auditory and Language
Acetylcholinesterase Staining in Human Jeffrey J. Hutsler and Michael S. Gazzaniga Auditory and Language Cortices: Center for Neuroscience, University of California, Davis Regional Variation of Structural Features California 95616 Cholinergic innovation of the cerebral neocortex arises from the basal 1974; Greenfield, 1984, 1991; Robertson, 1987; Taylor et al., forebrain and projects to all cortical regions. Acetylcholinesterase 1987; Krisst, 1989; Small, 1989, 1990). AChE-containing axons (AChE), the enzyme responsible for deactivating acetylcholine, is found of the cerebral cortex are also immunoreactive for choline within both cholinergic axons arising from the basal forebrain and a acetyltransferase (ChAT) and are therefore known to be cho- Downloaded from subgroup of pyramidal cells in layers III and V of the cerebral cortex. linergic (Mesulam and Geula, 1992). This pattern of staining varies with cortical location and may contrib- AChE-containing pyramidal cells of layers III and V are not ute uniquely to cortical microcircuhry within functionally distinct cholinergic (Mesulam and Geula, 1991), but it has been sug- regions. To explore this issue further, we examined the pattern of AChE gested that they are cholinoceptive (Krnjevic and Silver, 1965; staining within auditory, auditory association, and putative language Levey et al., 1984; Mesulam et al., 1984b). In support of this regions of whole, postmortem human brains. notion, layer in and V pyramidal cell excitability can be mod- http://cercor.oxfordjournals.org/ The density and distribution of acetylcholine-containing axons and ulated by the application of acetylcholine in the slice prepa- pyramidal cells vary systematically as a function of auditory process- ration (McCormick and Williamson, 1989). Additionally, mus- ing level. -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem. -
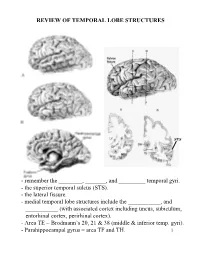
Review of Temporal Lobe Structures
REVIEW OF TEMPORAL LOBE STRUCTURES STS - remember the ________, _______, and _________ temporal gyri. - the superior temporal sulcus (STS). - the lateral fissure. - medial temporal lobe structures include the ___________, and ___________ (with associated cortex including uncus, subiculum, entorhinal cortex, perirhinal cortex). - Area TE = Brodmann’s 20, 21 & 38 (middle & inferior temp. gyri). - Parahippocampal gyrus = area TF and TH. 1 TEMPORAL LOBE FUNCTIONS Sensory Inputs to Temporal lobe: 1. ____________________________________________; 2. _________________________________________________. Temporal cortical regions and functional correlates: 1. Within lateral fissure (superior surface of lateral fissure): a) Heschel’s gyri (_____________________). b) posterior to Heschel’s gyri (_______________________). c) Planum temporale (secondary auditory cortex; Wernicke’s area - specialized in __________________________). 2 Temporal cortical regions and functional correlates (continued): 2. Superior temporal sulcus, middle and inferior temporal gyrus (area TE): _________________________________________ ____________. 3. Ventral/medial surface of temporal lobe (hippocampus and associated cortex): ______________________________. - the ventral/medial surface of the temporal lobe is also associated with the amygdala. Together with the surrounding ventral/medial temporal lobe, the amygdala is involved in __________________________________________. Hemispheric “specialization”: 1. Left hemisphere: a) ________________; b) ____________________________. -

Microsurgical and Tractographic Anatomical Study of Insular and Transsylvian Transinsular Approach
Neurol Sci (2011) 32:865–874 DOI 10.1007/s10072-011-0721-2 ORIGINAL ARTICLE Microsurgical and tractographic anatomical study of insular and transsylvian transinsular approach Feng Wang • Tao Sun • XinGang Li • HeChun Xia • ZongZheng Li Received: 29 September 2008 / Accepted: 16 July 2011 / Published online: 24 August 2011 Ó The Author(s) 2011. This article is published with open access at Springerlink.com Abstract This study is to define the operative anatomy of Introduction the insula with emphasis on the transsylvian transinsular approach. The anatomy was studied in 15 brain specimens, In humans, the insula is a highly developed structure, among five were dissected by use of fiber dissection totally encased within the brain. In many clinical and technique; diffusion tensor imaging of 10 healthy volun- experimental studies, a variety of functions have been teers was obtained with a 1.5-T MR system. The temporal attributed to the insula, however, the full and comprehen- stem consists mainly of the uncinate fasciculus, inferior sive role that it plays continues to remain obscure. Oper- occipitofrontal fasciculus, Meyer’s loop of the optic radi- ation of neurosurgery, specifically of epilepsy surgery, is a ation and anterior commissure. The transinsular approach window onto function and dysfunction of the human brain requires an incision of the inferior limiting sulcus. In this [1]. The insula, as part of the paralimbic system, has both procedure, the fibers of the temporal stem can be inter- invasive anatomical and functional connections with the rupted to various degrees. The fiber dissection technique is temporal lobe through white matter fibers [2–6]. -

Altered Resting State Connectivity of the Insular Cortex in Individuals with Fibromyalgia
The Journal of Pain, Vol 15, No 8 (August), 2014: pp 815-826 Available online at www.jpain.org and www.sciencedirect.com Original Reports Altered Resting State Connectivity of the Insular Cortex in Individuals With Fibromyalgia Eric Ichesco,* Tobias Schmidt-Wilcke,*,** Rupal Bhavsar,*,y Daniel J. Clauw,* Scott J. Peltier,z Jieun Kim,x Vitaly Napadow,x,{,k Johnson P. Hampson,* Anson E. Kairys,*,yy David A. Williams,* and Richard E. Harris* *Department of Anesthesiology, Chronic Pain and Fatigue Research Center, University of Michigan, Ann Arbor, Michigan. yNeurology Department, University of Pennsylvania, Philadelphia, Pennsylvania. z Functional MRI Laboratory, University of Michigan, Ann Arbor, Michigan. xMGH/MIT/HMS Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, Massachusetts. {Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts. kDepartment of Radiology, Logan College of Chiropractic, Chesterfield, Missouri. **Department of Neurology, Bergmannsheil, Ruhr University Bochum, Bochum, Germany. yyDepartment of Psychology, University of Colorado Denver, Denver, Colorado. Abstract: The insular cortex (IC) and cingulate cortex (CC) are critically involved in pain perception. Previously we demonstrated that fibromyalgia (FM) patients have greater connectivity between the insula and default mode network at rest, and that changes in the degree of this connectivity were associated with changes in the intensity of ongoing clinical pain. In this study we more thoroughly evaluated the degree of resting-state connectivity to multiple regions of the IC in individuals with FM and healthy controls. We also investigated the relationship between connectivity, experimental pain, and current clinical chronic pain. Functional connectivity was assessed using resting-state functional magnetic resonance imaging in 18 FM patients and 18 age- and sex-matched healthy controls using predefined seed regions in the anterior, middle, and posterior IC. -

A Brief Anatomical Sketch of Human Ventromedial Prefrontal Cortex Jamil P
This article is a supplement referenced in Delgado, M. R., Beer, J. S., Fellows, L. K., Huettel, S. A., Platt, M. L., Quirk, G. J., & Schiller, D. (2016). Viewpoints: Dialogues on the functional role of the ventromedial prefrontal cortex. Nature Neuroscience, 19(12), 1545-1552. Brain images used in this article (vmPFC mask) are available at https://identifiers.org/neurovault.collection:5631 A Brief Anatomical Sketch of Human Ventromedial Prefrontal Cortex Jamil P. Bhanji1, David V. Smith2, Mauricio R. Delgado1 1 Department of Psychology, Rutgers University - Newark 2 Department of Psychology, Temple University The ventromedial prefrontal cortex (vmPFC) is a major focus of investigation in human neuroscience, particularly in studies of emotion, social cognition, and decision making. Although the term vmPFC is widely used, the zone is not precisely defined, and for varied reasons has proven a complicated region to study. A difficulty identifying precise boundaries for the vmPFC comes partly from varied use of the term, sometimes including and sometimes excluding ventral parts of anterior cingulate cortex and medial parts of orbitofrontal cortex. These discrepancies can arise both from the need to refer to distinct sub-regions within a larger area of prefrontal cortex, and from the spatially imprecise nature of research methods such as human neuroimaging and natural lesions. The inexactness of the term is not necessarily an impediment, although the heterogeneity of the region can impact functional interpretation. Here we briefly address research that has helped delineate sub-regions of the human vmPFC, we then discuss patterns of white matter connectivity with other regions of the brain and how they begin to inform functional roles within vmPFC.