(PHA) Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preferred Name
Cord 2006, 22 (1) Cocoa pod borer threatening the sustainable coconut-cocoa cropping system in Papua New Guinea S. P. Singh¹ and P. Rethinam¹ Abstract Cocoa pod borer Conopomorpha cramerella Snellen is an important pest of cocoa. Its recent invasion of cocoa in Papua New Guinea is threatening the sustainable coconut-cocoa cropping system. C. cramerella occurs only in South-East Asia and the western Pacific. It has been the single most important limiting factor to cocoa production in Indonesia, Malaysia and the Philippines. C. cramerella attacks cocoa, Theobroma cacao; rambutan, Nephelium lappaceum; cola, Cola acuminate; pulasan, Nephelium mutabile; kasai, Albizia retusa, nam-nam,Cynometra cauliflora; litchi (Litchi chinensis); longan (Dimocarpus longan) and taun, Pometia pinnata. The possible mode of its spread is through seed pods/fruits carried from previously infested regions. However, there is possibility of local adaptations of rambutan-feeders or nam-nam-feeders to cocoa. Females lay their eggs (50-100) on the surface of the unripe pods (more than five cm in length), which hatch in about 3-7 days and the emerging larvae tunnel their way to the center of the pod where they feed for about 14-18 days before chewing their way out of the pod to pupate. The pupal stage lasts 6- 8 days. The larval feeding results in pods that may ripen prematurely, with small, flat beans, often stuck together in a mass of dried mucilage. The beans from seriously infested pods are completely unusable and in heavy infestation over half the potential crop can be lost. Over four dozen species of natural enemies (parasitoids, predators, and entomopathogens) attack eggs, larvae, pupae and adults of C. -
Additional File 1
Additional file 1 Microbial succession during the transition from active to inactive stages of deep-sea hydrothermal vent sulfide chimneys Jialin Hou, Stefan M. Sievert, Yinzhao Wang, Jeff S. Seewald, Vengadesh Perumal Natarajan Fengping Wang*, Xiang Xiao* a b 100 100 75 75 Taxa Alphaproteobacteria Taxa Betaproteobacteria Aquificae Deinococcus-Thermus Campylobacteria Deltaproteobacteria 50 Euryarchaeata 50 Euryarchaeata Others Gammaproteobacteria Percentage Percentage Thermodesulfobacterium Muproteobacteria Unclassified Nitrospirae Others Unclassified 25 25 0 0 aclA aclB cdhA cdhC napA napB norB nosZ sat soxA soxC soxY soxZ sqr aprA aprB dsrA dsrB narG nirB PRK rbcL rbcS Gene Gene Figure S1 Taxonomic classification of key functional genes retrieved from the L- and M-vent chimeny.(a) The key genes enriched in the active L-vent chimney. (b) The key genes enriched in the recently inactive M-vent chimney. Reductive bacterial type Reductive archaeal type Chlorobi group Archaeoglobus(3) Oxidative bacterial type Crenarchaeota Alphaproteobacteria(1/16) Nitrospirae(12) Acidobacteria(1/14) Betaproteobacteria Firmicutes groups Gammaproteobacteria (20) Tree scale: 1 Deltaproteobacteria(9/10) Reductive bacterial type Figure S2 Maximum-likelihood phylogeny of dsrA genes retrieved from L- and M-vent chimney. The red branches represent the dsrA genes recovered from active the L-vent chimney, while the blue ones are those from recently inactive M-vent chimney. Numbers of dsrA gene for each sample are displayed in the parenthesis after the clade name. -

Spore Strips, Crushable S
303-987-8000 or 800-992-6372 [email protected] Regulatory officials and sterilization experts have voiced concerns regarding the appropriateness of using a Biological Indicator (BI) Ampoule interchangeably with spore strips or other approved self-contained Biological Indicators (BIs). They argued spores in a sealed glass ampoule do not have direct contact with the steam, and this lack of direct contact with the sterilant caused the Ampoule to behave differently than other types of BIs. There was no scientific data to support this argument, only the belief that since the spores do not have direct contact with the steam, the Ampoule should not be used in porous load cycles because a “poor quality steam environment” might not be detected by the Ampoule. This argument disregards the fact that the Ampoule BIs are tested for population, Dvalue and Zvalue by the same standardized methods and equipment that are used to test other BIs. The following report will describe various tests and data collected to determine if the Ampoule BI behaves equivalently to spore strips and other self-contained BIs. Background: Biological Indicators (BIs) are used to determine whether a sterilizer has delivered a lethal cycle. Evaluation of resistant, spore-forming microorganisms processed through steam cycles gives the operator a direct measurement of the lethality delivered by the sterilizer during that particular cycle. The organisms used are of known quantity (population) and resistance (Dvalue). The organisms are packaged in such a way as to allow the sterilant access to the spores, and allow for either enumeration or recovery of surviving organisms. -

Simultaneous Determination of Arsenic, Manganese and Selenium in Human Serum by Neutron Activation Analysis
View metadata,Downloaded citation and from similar orbit.dtu.dk papers on:at core.ac.uk Dec 20, 2017 brought to you by CORE provided by Online Research Database In Technology Simultaneous determination of arsenic, manganese and selenium in human serum by neutron activation analysis Damsgaard, E.; Heydorn, Kaj; Larsen, N.A.; Nielsen, B. Publication date: 1973 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Damsgaard, E., Heydorn, K., Larsen, N. A., & Nielsen, B. (1973). Simultaneous determination of arsenic, manganese and selenium in human serum by neutron activation analysis. (Denmark. Forskningscenter Risoe. Risoe-R; No. 271). General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Risø Report No. 271 O Z 8o* Danish Atomic Energy Commission Bh Research Establishment Risø Simultaneous Determination of Arsenic, Manganese and Selenium in Human Serum by Neutron Activation Analysis by E. -
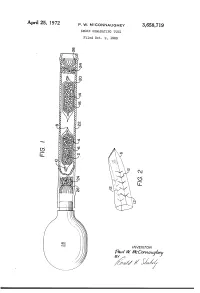
0M Mm EC Vc WM W
APII'I'l 25, 1972 P. w. MCCONNAUGHEY 3,658,719 SMOKE GENE-RATING TUBE Filed Oct. 9, 1969 \\\\\\\\\\~\ NJmyj \\\\\\\\\\\\\\\\\~\\\\\\\\\\\~Y \ R v.a la W. WMVc EC mm 0m (like), 1/17/; // - 1/ 1 .. 3,658,719 United States Patent 0 1C€ Patented Apr. 25, 1972 1 2 a perforated envelope 6 of polyethylene tubing heat 3,658,719 sealed at one end 8 and folded over at the other end 10. SMOKE GENERATING TUBE Paul W. McConnaughey, Wilkinsburg, Pa., assignor to As best seen in FIG. 2, the envelope has a plurality of Mine Safety Appliances Company, Pittsburgh, Pa. perforations 12 made as by cutting slits in the tubing Filed Oct. 9, 1969, Ser. No. 865,039 Wall. The perforations provide only small dimension Int. Cl. B01d; B01f; B01j 13/00 openings so that transfer of the volatile acid portion of US. Cl. 252—-359 A 2 Claims the reagent is substantially by diffusion; that is, there is no signi?cant convection ?ow of gases through the en velope. A great variety of methods of making suitable ABSTRACT OF THE DISCLOSURE 10 perforations are well known, such as, for example, slit ting or puncturing with needles or electrical sparks. Base A solid acid reagent and a solid base reagent are sepa reagent 14 is likewise contained in a breakable ampoule rately contained in a breakable ampoule that is enclosed 16 enclosed in perforated envelope 20. Both envelopes in a perforated envelope, which is in turn contained in are contained in a pliable tube 22 with suitable porous a pliable tube. -

Submission of Evidence Guidelines-101508-Print
GUIDELINES FOR THE COLLECTION AND SUBMISSION OF FORENSIC EVIDENCE Delaware Department of Health and Social Services Office of the Chief Medical Examiner Forensic Sciences Laboratory 200 South Adams St., Wilmington, DE 19801 (302)-577-3420 © Copyright 2008 Richard T. Callery, M.D., F.C.A.P. , Chief Medical Examiner and Director of the Office of the Chief Medical Examiner Forensic Sciences Laboratory Mission Statement The OCME evidentiary guidelines are dedicated to all past, present, and future public servants who dedicate their careers to providing the state of Delaware with the highest degree of law enforcement, forensic science, and medical-legal death investigation services while maintaining the traditions of fairness, professionalism, and integrity. Delaware OCME - Forensic Sciences Laboratory Evidence Submission Guidelines 2008 Rev (0).pub — Page 3 — TABLE OF CONTENTS Introduction············································································································ 7 Using the Laboratory in the Judicial Process···························································· 8 Crime Scene Processing························································································· 9 General Submission Instructions ·······································································11-15 General Information·························································································· 11 Choosing Containers ························································································ -

WSU SURCA Poster Event Leads to 51 Awards to 57 Undergraduate Researchers
March 27, 2017 SURCA.WSU.EDU SURCA is hosted by the Office of Undergraduate Research part of WSU Undergraduate Education. TABLE OF CONTENTS Schedule of Events .................................................................... 2 SURCA 2017 Committee .......................................................... 3 Judges (alphabetical listing) ...................................................... 4 Judges (external organizations) ................................................ 5 Judging Rubric .......................................................................... 6 Sponsors .................................................................................... 7 Award Winners (news article) ................................................... 8 Entries (presenters in alphabetical order) ............................... 12 Abstracts (numerically by presentation number) .................. 34 1 SCHEDULE OF EVENTS Monday, March 27, 2017 Posters: M.G. Carey Senior Ballroom, Compton Union Building (CUB) Awards: CUB Auditorium (Room 177) Noon – 2 p.m. Student presenters hang their own posters 2:00 - 2:45 p.m. Informal judging (no students present) 2:45 – 3:45 p.m. Formal judging (only judges and presenters in room until 3:30 p.m.) 3:30 – 5:00 p.m. Public viewing 5:00 – 5:45 p.m. SURCA Awards Ceremony (all welcome to attend) 5:45 p.m. Presenters remove posters and pick up judges’ feedback sheets 2 COMMITTEE Talea Anderson WSU Libraries Lydia Gerber College of Arts and Sciences Samantha Gizerian College of Veterinary Medicine Kaitlin Hennessy Global -

VSMOW Triple Point of Water Cells: Borosilicate Versus Fused-Quartz
VSMOW Triple Point of Water Cells: Borosilicate versus Fused- Quartz M. Zhao1,3 and G. F. Strouse 2 1 Fluke Corporation, Hart Scientific Division, American Fork, Utah 84003, U.S.A. 2 National Institute of Standards and Technology, Gaithersburg, Maryland 20899, U.S.A. 3 To whom correspondence should be addressed. E-mail: [email protected] ABSTRACT To investigate an ideal container material for the triple point of water (TPW) cell, and reduce the influence to the triple-point temperature due to the deviation of the isotopic composition of the water, we developed and tested both borosilicate and fused-quartz glass shelled TPW cells with isotopic composition substantially matching that of Vienna Standard Mean Ocean Water (VSMOW). Through a specially designed manufacturing system, the isotopic composition, δD and δ18O, of the water in the TPW cell could be controlled within ±10‰ (per mil) and ±1.5‰ respectively, resulting in control of the isotopic temperature correction to better than ±8 µK. Through an ampoule attached to the cell, the isotopic composition of the water in the cell could be analyzed individually. After manufacture, the initial triple-point temperature of the two types of cell were measured and compared to assess the quality of the cells and manufacturing process. Cells fabricated with the new system agree to within 50 µK. Two innovatively-designed borosilicate and fused-quartz TPW cells were made, each with six attached ampoules. We removed one ampoule every six months to track any changes in purity of the water over time. KEY WORDS: isotopic composition; ITS-90, TPW cell; Vienna standard mean ocean water; VSMOW; water impurities; water triple point. -

Caldimonas Taiwanensis Sp. Nov., a Amylase Producing Bacterium
ARTICLE IN PRESS Systematic and Applied Microbiology 28 (2005) 415–420 www.elsevier.de/syapm Caldimonas taiwanensis sp. nov., a amylase producing bacterium isolated from a hot spring Wen-Ming Chena,Ã, Jo-Shu Changb, Ching-Hsiang Chiua, Shu-Chen Changc, Wen-Chieh Chena, Chii-Ming Jianga aDepartment of Seafood Science, National Kaohsiung Marine University, No. 142, Hai-Chuan Rd. Nan-Tzu, Kaohsiung City 811, Taiwan bDepartment of Chemical Engineering, National Cheng Kung University, Tainan, Taiwan cTajen Institute of Technology, Yen-Pu, Pingtung, Taiwan Received 22 February 2005 Abstract During screening for amylase-producing bacteria, a strain designated On1T was isolated from a hot spring located in Pingtung area, which is near the very southern part of Taiwan. Cells of this organism were Gram-negative rods motile by means of a single polar flagellum. Optimum conditions for growthwere 55 1C and pH 7. Strain On1T grew well in minimal medium containing starchas thesole carbon source, and its extracellular products expressed amylase activity. The 16S rRNA gene sequence analysis indicates that strain On1T is a member of b-Proteobacteria. On the basis of a phylogenetic analysis of 16S rDNA sequences, DNA–DNA similarity data, physiological and biochemical characteristics, as well as fatty acid compositions, the organism belonged to the genus Caldimonas and represented a novel species within this genus. The predominant cellular fatty acids of strain On1T were 16:0 (about 30%), 18:1 o7c (about 20%) and summed feature 3 (16:1o7c or 15:0 iso 2OH or both[about 31%]). Its DNA base ratio was 65.9 mol% G+C. -

"Sodium Chloride," In
Article No : a24_317 Article with Color Figures Sodium Chloride GISBERT WESTPHAL, Solvay Salz GmbH, Solingen, Federal GERHARD KRISTEN, Solvay Salz GmbH, Solingen, Federal WILHELM WEGENER, Sudwestdeutsche€ Salzwerke AG, Heilbronn, Germany PETER AMBATIELLO, Salzbergwerk Berchtesgaden, Germany HELMUT GEYER, Salzgewinnungsgesellschaft Westfalen mbH, Ahaus, Germany BERNARD EPRON, Compagnie des Salins du Midi et des Salines de l’Est, Paris, France CHRISTIAN BONAL, Compagnie des Salins du Midi et des Salines de l’Est, Paris, France ¨ GEORG STEINHAUSER, Atominstitut der Osterreichischen Universit€aten, Vienna University of Technology, Austria FRANZ Go€TZFRIED, Sudsalz€ GmbH, Heilbronn, Germany 1. History ..........................319 4.3.6. Other Process Steps ...................343 2. Properties ........................320 4.3.7. Evaluation of the Different Processes ......344 3. Formation and Occurrence of 4.3.8. Vacuum Salt based on Seawater as Raw Salt Deposits ......................322 Material . ........................345 4. Production ........................324 4.4. Production of Solar Salt...............346 4.1. Mining of Rock Salt from Underground 4.4.1. Production from Sea Water . ...........346 and Surface Deposits .................324 4.4.1.1. The Main Factors Governing Production of 4.1.1. Mining by Drilling and Blasting..........325 Sea Salt . ........................347 4.1.2. Continuous Mining ...................327 4.4.1.2. Production Stages in a Modern Salt Field . .349 4.1.3. Upgrading of Rock Salt . ...............327 4.4.2. Crystallization from Mined Brine . ......351 4.1.4. Utilization of the Chambers . ...........329 4.4.3. Extraction from Salt Lakes. ...........351 4.2. Brine Production ....................330 4.4.4. Seawater Desalination . ...............352 4.2.1. Natural Brine Extraction ...............330 5. Salt Standards .....................352 4.2.2. -

Land Reclamation and Artificial Islands
Global Ecology and Conservation 12 (2017) 80e95 Contents lists available at ScienceDirect Global Ecology and Conservation journal homepage: http://www.elsevier.com/locate/gecco Original Research Article Land reclamation and artificial islands: Walking the tightrope between development and conservation Su Yin Chee a, *, Abdul Ghapar Othman b, Yee Kwang Sim a, Amni Nabilah Mat Adam a, Louise B. Firth c a Centre for Marine and Coastal Studies, Universiti Sains Malaysia, 11800, Minden, Malaysia b School of Housing, Building and Planning, Universiti Sains Malaysia, 11800, Minden, Malaysia c School of Biological and Marine Sciences, Plymouth University, Drake Circus, Plymouth, PL4 8AA, UK article info abstract Article history: Coastal developments worldwide have put entire shoreline ecosystems at risk. Recently, Received 31 July 2017 land reclamation has been extended to the construction of whole new islands; a phe- Accepted 13 August 2017 nomenon that is particularly common in Asia and the Middle East and is recognised as a global conservation issue. Using Penang Island, Malaysia as a case study, we illustrate the relationship between rapid population growth and the simultaneous increase in urbani- Keywords: sation, land reclamation and extent of artificial shorelines; and decrease in the quality and Coastal management extent of natural coastal habitats. Our goal was to provide an up-to-date assessment of the Ecological engineering Erosion state of coastal habitats around Penang, identify knowledge gaps and identify locations Mangrove that may be potentially suitable for eco-engineering. Comparisons of historical and current Ocean sprawl topographic maps revealed that land formerly consisting of coastal swamp and forest, Sustainable coastal development mangrove forests, sandy beaches, and rubber and oil plantations have been lost to large- Urbanisation scale land reclamation and urbanisation. -

Cecilia Gonzales Marin
MOLECULAR DETECTION OF BACTERIA FROM A POSSIBLE MATERNAL ORAL ORIGIN IN NEONATAL GASTRIC ASPIRATES OBTAINED FROM COMPLICATED PREGNANCIES Thesis submitted to the University of London to obtain the degree of DOCTOR OF PHILOSOPHY Cecilia Gonzales Marin Institute of Dentistry Barts and The London School of Medicine and Dentistry Queen Mary, University of London 2011 SUPERVISORS: Rob Allaker, PhD Queen Mary University of London Barts and The London School of Medicine and Dentistry Centre for Clinical and Diagnostic Oral Sciences David Spratt, PhD University College London Eastman Dental Institute Division of Microbial Diseases 2 ABSTRACT It has been suggested that periodontal disease, a disease that affects the supporting tissues of the teeth, represents a risk factor for adverse pregnancy outcomes. Certain oral pathogens possess a demonstrated ability to translocate and invade the amniotic tissues. Once in the amniotic environment, these opportunistic colonisers could then initiate or contribute to a perinatal infection, and in this way be involved in the complications. The overall aim of this study was to determine the presence, and confirm the origin, of suspected maternal oral microbiota in neonatal gastric aspirates (swallowed amniotic fluid) collected due to complications during pregnancy and/or evidence of neonatal sepsis. Non-cultural PCR-based methods directed to the ribosomal encoding genes (rDNA) were applied to analyse neonatal and maternal samples. The use of universal and species-specific primers that target the bacterial 16S rRNA gene allowed identification and quantification of broad-range and specific bacteria to the species level. Sequence comparative analysis of a more variable fragment, the intergenic spacer region located between the 16S and the 23S rDNA, was finally used to compare strains obtained from the neonates and their counterparts in the respective mother’s oral and vaginal samples.