VSMOW Triple Point of Water Cells: Borosilicate Versus Fused-Quartz
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermal Shock
TEACHER INSTRUCTIONS Thermal Shock Objective: To illustrate thermal expansion and thermal shock. Background Information: In physics, thermal expansion is the tendency of matter to increase in volume or pressure when heated. For liquids and solids, the amount of expansion will normally vary depending on the material’s coefficient of thermal expansion. When materials contract, tensile forces are created. When things expand, compressive forces are created. Thermal shock is the name given to cracking as a result of rapid temperature change. From the laboratory standpoint, there are three main types of glass used today: borosilicate, quartz, and soda lime or flint glass. Borosilicate glass is made to withstand thermal shock better than most other glass through a combination of reduced expansion coefficient and greater strength, though fused quartz outperforms it in both respects. Some glass-ceramic materials include a controlled proportion of material with a negative expansion coefficient, so that the overall coefficient can be reduced to almost exactly zero over a reasonably wide range of temperatures. Improving the shock resistance of glass and ceramics can be achieved by improving the strength of the materials or by reducing its tendency to uneven expansion. One example of success in this area is Pyrex, the brand name that is well known to most consumers as cookware, but which is also used to manufacture laboratory glassware. Pyrex traditionally is made with a borosilicate glass with the addition of boron, which prevents shock by reducing the tendency of glass to expand. Demo description: Three different types of glass rods will be heated so that students can observe the amount of thermal shock that occurs. -

Spore Strips, Crushable S
303-987-8000 or 800-992-6372 [email protected] Regulatory officials and sterilization experts have voiced concerns regarding the appropriateness of using a Biological Indicator (BI) Ampoule interchangeably with spore strips or other approved self-contained Biological Indicators (BIs). They argued spores in a sealed glass ampoule do not have direct contact with the steam, and this lack of direct contact with the sterilant caused the Ampoule to behave differently than other types of BIs. There was no scientific data to support this argument, only the belief that since the spores do not have direct contact with the steam, the Ampoule should not be used in porous load cycles because a “poor quality steam environment” might not be detected by the Ampoule. This argument disregards the fact that the Ampoule BIs are tested for population, Dvalue and Zvalue by the same standardized methods and equipment that are used to test other BIs. The following report will describe various tests and data collected to determine if the Ampoule BI behaves equivalently to spore strips and other self-contained BIs. Background: Biological Indicators (BIs) are used to determine whether a sterilizer has delivered a lethal cycle. Evaluation of resistant, spore-forming microorganisms processed through steam cycles gives the operator a direct measurement of the lethality delivered by the sterilizer during that particular cycle. The organisms used are of known quantity (population) and resistance (Dvalue). The organisms are packaged in such a way as to allow the sterilant access to the spores, and allow for either enumeration or recovery of surviving organisms. -

Simultaneous Determination of Arsenic, Manganese and Selenium in Human Serum by Neutron Activation Analysis
View metadata,Downloaded citation and from similar orbit.dtu.dk papers on:at core.ac.uk Dec 20, 2017 brought to you by CORE provided by Online Research Database In Technology Simultaneous determination of arsenic, manganese and selenium in human serum by neutron activation analysis Damsgaard, E.; Heydorn, Kaj; Larsen, N.A.; Nielsen, B. Publication date: 1973 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Damsgaard, E., Heydorn, K., Larsen, N. A., & Nielsen, B. (1973). Simultaneous determination of arsenic, manganese and selenium in human serum by neutron activation analysis. (Denmark. Forskningscenter Risoe. Risoe-R; No. 271). General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Risø Report No. 271 O Z 8o* Danish Atomic Energy Commission Bh Research Establishment Risø Simultaneous Determination of Arsenic, Manganese and Selenium in Human Serum by Neutron Activation Analysis by E. -
0M Mm EC Vc WM W
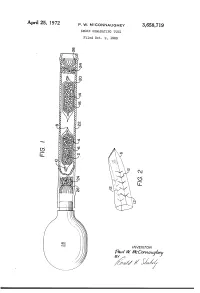
APII'I'l 25, 1972 P. w. MCCONNAUGHEY 3,658,719 SMOKE GENE-RATING TUBE Filed Oct. 9, 1969 \\\\\\\\\\~\ NJmyj \\\\\\\\\\\\\\\\\~\\\\\\\\\\\~Y \ R v.a la W. WMVc EC mm 0m (like), 1/17/; // - 1/ 1 .. 3,658,719 United States Patent 0 1C€ Patented Apr. 25, 1972 1 2 a perforated envelope 6 of polyethylene tubing heat 3,658,719 sealed at one end 8 and folded over at the other end 10. SMOKE GENERATING TUBE Paul W. McConnaughey, Wilkinsburg, Pa., assignor to As best seen in FIG. 2, the envelope has a plurality of Mine Safety Appliances Company, Pittsburgh, Pa. perforations 12 made as by cutting slits in the tubing Filed Oct. 9, 1969, Ser. No. 865,039 Wall. The perforations provide only small dimension Int. Cl. B01d; B01f; B01j 13/00 openings so that transfer of the volatile acid portion of US. Cl. 252—-359 A 2 Claims the reagent is substantially by diffusion; that is, there is no signi?cant convection ?ow of gases through the en velope. A great variety of methods of making suitable ABSTRACT OF THE DISCLOSURE 10 perforations are well known, such as, for example, slit ting or puncturing with needles or electrical sparks. Base A solid acid reagent and a solid base reagent are sepa reagent 14 is likewise contained in a breakable ampoule rately contained in a breakable ampoule that is enclosed 16 enclosed in perforated envelope 20. Both envelopes in a perforated envelope, which is in turn contained in are contained in a pliable tube 22 with suitable porous a pliable tube. -

Submission of Evidence Guidelines-101508-Print
GUIDELINES FOR THE COLLECTION AND SUBMISSION OF FORENSIC EVIDENCE Delaware Department of Health and Social Services Office of the Chief Medical Examiner Forensic Sciences Laboratory 200 South Adams St., Wilmington, DE 19801 (302)-577-3420 © Copyright 2008 Richard T. Callery, M.D., F.C.A.P. , Chief Medical Examiner and Director of the Office of the Chief Medical Examiner Forensic Sciences Laboratory Mission Statement The OCME evidentiary guidelines are dedicated to all past, present, and future public servants who dedicate their careers to providing the state of Delaware with the highest degree of law enforcement, forensic science, and medical-legal death investigation services while maintaining the traditions of fairness, professionalism, and integrity. Delaware OCME - Forensic Sciences Laboratory Evidence Submission Guidelines 2008 Rev (0).pub — Page 3 — TABLE OF CONTENTS Introduction············································································································ 7 Using the Laboratory in the Judicial Process···························································· 8 Crime Scene Processing························································································· 9 General Submission Instructions ·······································································11-15 General Information·························································································· 11 Choosing Containers ························································································ -

WSU SURCA Poster Event Leads to 51 Awards to 57 Undergraduate Researchers
March 27, 2017 SURCA.WSU.EDU SURCA is hosted by the Office of Undergraduate Research part of WSU Undergraduate Education. TABLE OF CONTENTS Schedule of Events .................................................................... 2 SURCA 2017 Committee .......................................................... 3 Judges (alphabetical listing) ...................................................... 4 Judges (external organizations) ................................................ 5 Judging Rubric .......................................................................... 6 Sponsors .................................................................................... 7 Award Winners (news article) ................................................... 8 Entries (presenters in alphabetical order) ............................... 12 Abstracts (numerically by presentation number) .................. 34 1 SCHEDULE OF EVENTS Monday, March 27, 2017 Posters: M.G. Carey Senior Ballroom, Compton Union Building (CUB) Awards: CUB Auditorium (Room 177) Noon – 2 p.m. Student presenters hang their own posters 2:00 - 2:45 p.m. Informal judging (no students present) 2:45 – 3:45 p.m. Formal judging (only judges and presenters in room until 3:30 p.m.) 3:30 – 5:00 p.m. Public viewing 5:00 – 5:45 p.m. SURCA Awards Ceremony (all welcome to attend) 5:45 p.m. Presenters remove posters and pick up judges’ feedback sheets 2 COMMITTEE Talea Anderson WSU Libraries Lydia Gerber College of Arts and Sciences Samantha Gizerian College of Veterinary Medicine Kaitlin Hennessy Global -

Ultradeep Fused Silica Glass Etching with an HF- Resistant Photosensitive Resist for Optical Imaging Applications
Ultradeep fused silica glass etching with an HF- resistant photosensitive resist for optical imaging applications John M Nagarah and Daniel A Wagenaar Broad Fellows Program and Division of Biology California Institute of Technology 1200 E. California Blvd. MC 216-76 Pasadena, CA 91125 [email protected] [email protected] Abstract Microfluidic and optical sensing platforms are commonly fabricated in glass and fused silica (quartz) because of their optical transparency and chemical inertness. Hydrofluoric acid (HF) solutions are the etching media of choice for deep etching into silicon dioxide substrates, but processing schemes become complicated and expensive for etching times greater than 1 hour due to the aggressiveness of HF migration through most masking materials. We present here etching into fused silica more than 600 μm deep while keeping the substrate free of pits and maintaining a polished etched surface suitable for biological imaging. We utilize an HF-resistant photosensitive resist (HFPR) which is not attacked in 49% HF solution. Etching characteristics are compared for substrates masked with the HFPR alone and the HFPR patterned on top of Cr/Au and polysilicon masks. We used this etching process to fabricate suspended fused silica membranes, 8–16 μm thick, and show that imaging through the membranes does not negatively affect image quality of fluorescence microscopy of biological tissue. Finally, we realize small through-pore arrays in the suspended membranes. Such devices will have applications in planar electrophysiology platforms, especially where optical imaging is required. 1. Introduction Glass and fused silica are appealing materials for constructing microelectromechanical systems (MEMS), lab-on-a-chip, and microfluidic platforms due to their chemical inertness, biocompatibility, optical transparency, mechanical rigidity, high melting point, electrical insulation, gas impermeability, and ability to bond to silicon, glass, and polydimethylsiloxane (PDMS) [1-3]. -

Investigation of Light Output Uniformity and Performance Using a UV
Investigation of light output uniformity and performance using a UV transmitting glass optic to improve cure quality Brian Jasenak, Rachel Willsey, Adam Willsey, James Forish Kopp Glass 2108 Palmer Street Pittsburgh, PA 15218 ABSTRACT Ultraviolet light-emitting diode (UV LED) adoption is accelerating; they are being used in new applications such as UV curing, germicidal irradiation, nondestructive testing, and forensic analysis. In many of these applications, it is critically important to produce a uniform light distribution and consistent surface irradiance. Flat panes of fused quartz, silica, or glass are commonly used to cover and protect multi-UV LED arrays. However, they don’t offer the advantages of an optical lens design. An investigation was conducted to determine the effect of a secondary glass optic on the uniformity of the light distribution and irradiance. Glass optics capable of transmitting UV-A, UV-B, and UV-C wavelengths can improve light distribution and intensity. In this study, a UV transmitting glass formulation and secondary linear optic were designed and manufactured to demonstrate their effects on achievable irradiance intensity and uniformity. Prismatic patterning on the light source surface of the lens was used to minimize reflection losses on the incident surface of the glass. Fresnel optics were molded into the opposite side of the UV transmitting glass to control the refraction of the light and to gain the desired light intensity distribution from two multi-UV LED arrays. A 20% increase in relative irradiance was observed while maintaining the same coverage area. This work discusses the optical design and the resulting benefits of controlled light output on UV LED systems, which include reduced driving current, decreased thermal deterioration, improved energy efficiency, and longer LED lifetime. -

Specialty Glass Technical Capabilities
SpecialtySpecialty GlassGlass TechnicalTechnical CapabilitiesCapabilities 07/1307/13 Web: www.abrisatechnologies.com - E–mail: [email protected] - Tel: (877) 622-7472 Page 1 Specialty Glass Products Technical Reference Document 07/13 It all starts with the basic element, the glass. Each substrate has unique and specific qualities which are matched to the application and specifications that your unique project requires. Abrisa Technologies offers: High Ion-Exchange (HIE) Thin Glass High Ion-Exchange (HIE) Aluminosilicate Thin Glass - (Page 3) Asahi Dragontrail™ - (Pages 4 & 5) Corning® Gorilla® Glass - (Pages 6 & 7) SCHOTT Xensation™ Cover Glass - (Page 8) Soda-Lime Soda-Lime (Clear & Tinted) - (Page 9) Soda-Lime (Low Iron) - (Page 10) Soda-Lime (Anti-Glare Reducing Etched Glass) - (Page 11) Patterned Glass for Light Control - (Page 12 & 13) Soda-Lime Low Emissivity (Low-E) Glass - (Page 14) Soda-Lime (Heat Absorbing Float Glass) - (Page 15) Borosilicate SCHOTT BOROFLOAT® 33 Multi-functional Float Glass - (Pages 16 & 17) SCHOTT BOROFLOAT Infrared (IRR) - (Page 18) SCHOTT SUPREMAX® Rolled Borosilicate - (Pages 19 & 20) SCHOTT D263 Colorless Thin Glass - (Pages 21 & 22) SCHOTT Duran® Lab Glass - (Pages 24 & 25) Ceramic/Glass SCHOTT Robax® Transparent Ceramic Glass - (Page 26) SCHOTT Pyran® Fire Rated Glass Ceramic - (Page 27) Quartz/Fused Silica Corning® 7980 Fused Silica - (Page 28) GE 124 Fused Quartz - (Page 309 Specialty Glass Corning® Eagle XG LCD Glass Free of Heavy Metals - (Page 30 & 31) Laminated Glass - Safety Glass - (Page 32) SCHOTT Superwhite B270® Flat Glass - (Page 33) Weld Shield - (Page 354 White Flashed Opal - (Page 35) X-Ray Glass (Radiation Shielding Glass) - (Page 376 Web: www.abrisatechnologies.com - E–mail: [email protected] - Tel: (877) 622-7472 Page 2 Specialty Glass Products Technical Reference Document 07/13 High Ion-Exchange (HIE) High Ion-Exchange (HIE) Chemically Strengthened Aluminosilicate Thin Glass High Ion-Exchange (HIE) thin glass is strong, lightweight and flexible. -

Accessories for Differential Scanning Calorimeters and Thermobalances
Analyzing & Testing Accessories for Differential Scanning Calorimeters and Thermobalances Crucibles, Sensors, Sample Carriers, Calibration Kits for DSC, TGA and STA Systems Introduction – Table of Contents Accessories for Thermal Analysis – DSC/DTA, TGA and STA Thermal analysis is a powerful, well and instrument parts must be prevented meshes and baskets are available proven tool for obtaining reliable data while ensuring that the test results to accommodate specific sample on the caloric and thermophysical remain reliable and accurate. For these dimensions and densities. properties of a great variety of materials. reasons, one of our primary areas of focus is crucibles and sensors for DTA/ Lately, the demand for special crucibles To attain proper results, proficient DSC, TGA and STA instruments. has been increasing. Of course, state-of-the-art instruments are measurements can only be carried out required, featuring optimum technical This catalogue provides an overview of when the right sensor or sample carrier attributes such as high sensitivity and all such crucibles and sensors for DTA, for these special crucibles is available. resolution in the required temperature DSC, TGA and STA measurements. We have therefore listed these special range. In recent years, a rise in the You will find many different crucible cases here, often providing application development of new materials for materials listed, and a variety of types examples to demonstrate their emerging applications has been and special shapes. From among these, characteristic advantages. presenting an ongoing challenge for we can help you find the right crucible the thermal analysis industry in keeping size and material for any application, Our accessories can open up a world pace with rapidly evolving market be it standard or special. -

SUPTEV007 Poster Submission.Pdf
Development of a system for coating SRF cavities using Remote Plasma CVD (Chemical Vapor Deposition) Gabriel Gaitan, Zeming Sun, Adam Holic, Gregory Kulina, Matthias Liepe, James Sears, Paul Bishop (CLASSE) Setup Background • Samples are loaded on Moly boats and • Thin-film surfaces employing Nb3Sn, NbN, NbTiN, and introduced in the system for other compound superconductors are destined to annealing/CVD Clean Room allow reaching superior RF performance levels in SRF • Fused Quartz tube suitable to 1100C Turbopump cavities • An RF source will create plasma from the & VQM • Optimized, advanced deposition processes are precursors and this will reduce processing Quartz tube required to enable high-quality films of such materials temperature and promote precursor on large and complex shaped cavities. In doing this, decomposition Cornell University is developing a remote plasma- • Clean room is used for minimizing enhanced chemical vapor deposition (CVD) system contaminants in the furnace Furnace Furnace that facilitates coating on complicated geometries • Heat shields protect the O-rings on the controls with a high deposition rate. end flanges from overheating. Plasma source Cold trap Furnace offers independent control of heating for all 3 • Loading and unloading system is being designed to allow processing of small VQM is important for monitoring carbon and hydrogen zones of the furnace. Maximum furnace temperature samples and cavities of 2.6GHz and 3.9GHz. contamination that might affect cavity performance 1500C. Moly boats Maximum safe -

Production Line and Machines Automatic Tablet Press Production
Production Line and Machines Automatic Tablet Press Production Line Auto Coater Machines and Manuel Coating Machine Automatic Packaging Machines Full Automatic Filling Hard Capsule Production Line Full Automatic Filling Soft Gelatin Capsule Production Line Full Automatic Ampule Production Line Full Automatic Liqued Vial Production Line Full Automatic Powder Vial Production Line Full Automatic Liquid Syrup Production Line Full Automatic Dry Syrup Production Line Full Automatic Ointment Production Line Full Automatic Nasal Spray Filling Machine Full Automatic suppository Production Line Full Automatic I.V. Solution System Full Automatic Non-PVC Soft Bag IV Solution Production Line Full Automatic PP bottle IV solution production line Air Flow Drier AL-Plastic-AL Automatic Blister Packing Machine Aluminum Foil Ampoule Cleaning Machine Ampoule Compact Line Ampoule Filler Ampoule Filling and Sealing Machine Ampoule Filling Sealing Machine Ampoule Labeling Machine Ampoule Production Line Ampoule Sealing Machine Ampoule Sesler Ampoule Washer Ampoule Washing Machine Ampoule Washing, Drying and Filling Production Line Antibiotic Vial Washing Machine Aseptic Liquid Filling Machine Atuomatic Blister Packing Machine Atuomatic Liquid Vial Filling Machine Auto-Checking Forming Blister Packing Machine Automatic Air Jet Cleaning Machine Automatic Airjet & Vacuum Cleaning Machine Automatic Ampoule Filling & Sealing Machines Automatic Ampoule Inspection Machine Automatic Ampoule Screen Printing Machine Automatic Ampoule Sticker Labeling Machine Automatic