Analytical Methods and Procedures in the Small Winery Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Concepts in Wine Chemistry
THIRD EDITION Concepts IN Wine Chemistry YAIR MARGALIT, Ph.D. The Wine Appreciation Guild San Francisco Contents Introduction ix I. Must and Wine Composition 1 A. General Background 3 B. Sugars 5 C. Acids 11 D. Alcohols 22 E. Aldehydes and Ketones 30 F. Esters 32 G. Nitrogen Compounds 34 H. Phenols 43 I. Inorganic Constituents 52 References 55 n. Fermentation 61 A. General View 63 B. Chemistry of Fermentation 64 C. Factors Affecting Fermentation 68 D. Stuck Fermentation 77 E. Heat of Fermentation 84 F. Malolactic Fermentation 89 G. Carbonic Maceration 98 References 99 v III. Phenolic Compounds 105 A. Wine Phenolic Background 107 B. Tannins 120 C. Red Wine Color 123 D. Extraction of Phenolic Compounds from Grapes 139 References 143 IV. Aroma and Flavor 149 A. Taste 151 B. Floral Aroma 179 C. Vegetative Aroma 189 D. Fruity Aroma 194 E. Bitterness and Astringency 195 F. Specific Flavors 201 References 214 V. Oxidation and Wine Aging 223 A. General Aspects of Wine Oxidation 225 B. Phenolic Oxidation 227 C. Browning of White Wines 232 D. Wine Aging 238 References 253 VI. Oak Products 257 A. Cooperage 259 B. Barrel Aging 274 C. Cork 291 References 305 vi VH. Sulfur Dioxide 313 A. Sulfur-Dioxide as Food Products Preservative 315 B. Sulfur-Dioxide Uses in Wine 326 References 337 Vm. Cellar Processes 341 A. Fining 343 B. Stabilization 352 C. Acidity Adjustment 364 D. Wine Preservatives 372 References 382 IX. Wine Faults 387 A. Chemical Faults 389 B. Microbiological Faults 395 C. Summary ofFaults 402 References 409 X. -

Basic Definitions and Tips for Winemaking
Presque Isle Wine Cellars “Serving the Winemaker Since 1964” (814) 725-1314 www.piwine.com Basic Winemaking Terms & Tips Definitions & Tips: Not all-inclusive but hopefully helpful. Email us your favorites; maybe we’ll include them in the next edition. Acid Reduction - Reducing the acid in juice or wine to an acceptable level. It is usually measured as tartaric acid and requires a testing apparatus and reagents. Good levels are typically in a range of 0.6 to 0.8 percent acid, depending on the wine. More technically the reading is read as grams per liter. Therefore 0.6 percent would be 6.0 g/l. Acidulation or Acidification - Raising the acid level of juice, wine or sometimes water by adding some type of acid increasing additive or blending with a higher acid juice or wine. Acidified or Acidulated Water - Water to which acid (most commonly citric acid) has been added. It is a way to reduce sugar in a juice that is too high in sugar without diluting (thus reducing) the acid level of that juice. Additives - Things added to wine to enhance quality or possibly fix some type of flaw. There are many additives for many situations and it is wise to gain at least some basic knowledge in this area. Alcohol - Obviously one of the significant components of wine. Yeast turns sugar to alcohol. Rule of thumb says for each percentage of sugar in a non-fermented juice, the alcohol will be half. For example 21% sugar should ferment out to an alcohol level of about 11.5 to 12%. -

Spore Strips, Crushable S
303-987-8000 or 800-992-6372 [email protected] Regulatory officials and sterilization experts have voiced concerns regarding the appropriateness of using a Biological Indicator (BI) Ampoule interchangeably with spore strips or other approved self-contained Biological Indicators (BIs). They argued spores in a sealed glass ampoule do not have direct contact with the steam, and this lack of direct contact with the sterilant caused the Ampoule to behave differently than other types of BIs. There was no scientific data to support this argument, only the belief that since the spores do not have direct contact with the steam, the Ampoule should not be used in porous load cycles because a “poor quality steam environment” might not be detected by the Ampoule. This argument disregards the fact that the Ampoule BIs are tested for population, Dvalue and Zvalue by the same standardized methods and equipment that are used to test other BIs. The following report will describe various tests and data collected to determine if the Ampoule BI behaves equivalently to spore strips and other self-contained BIs. Background: Biological Indicators (BIs) are used to determine whether a sterilizer has delivered a lethal cycle. Evaluation of resistant, spore-forming microorganisms processed through steam cycles gives the operator a direct measurement of the lethality delivered by the sterilizer during that particular cycle. The organisms used are of known quantity (population) and resistance (Dvalue). The organisms are packaged in such a way as to allow the sterilant access to the spores, and allow for either enumeration or recovery of surviving organisms. -

Press-Kit-2015-CIVR
PRESS PACK 2015 THE WINES OF ROUSSILLON www.winesofroussillon.com / www.vinsduroussillon.com Contact Eric ARACIL [email protected] - 1 - For free use. GEOGRAPHICAL SITUATION 4 A LAND BLESSED BY THE GODS 5 THE LEGACY OF THE ANCIENT GREEKS 5 THE SPREAD OF EXPORTS 6 THE RAPID EXPANSION OF THE VINEYARD 6 THE ERA OF RECOGNITION 7 SUD DE FRANCE/SOUTH OF FRANCE 8 GENERAL INTRODUCTION TO THE VINEYARDS 9 14 AOP, 3 IGP AND 23 VARIETALS: EXTERNAL SIGNS OF THE WEALTH OF WINES 10 A SOCIETY OF SMALL WINE GROWERS 10 VARIED TERROIRS 11 A – TO THE NORTH WEST OF THE TÊT RIVER, 11 B – TO THE NORTH EAST OF THE TÊT RIVER 12 C - TO THE SOUTH OF THE TÊT RIVER 13 D- THE BANYULS AND COLLIOURE AREA 13 THE IDEAL CLIMATE 14 23 VARIETALS FOR PEDIGREE WINES 15 WHITE AND GREY VARIETALS 15 GRENACHE BLANC 15 GRENACHE GRIS 15 MACABEU 15 MALVOISIE DU ROUSSILLON BLANCHE 16 MARSANNE 16 MUSCAT D’ALEXANDRIE 17 MUSCAT A PETITS GRAINS 17 ROUSSANNE 17 VERMENTINO 18 BLACK VARIETAL 18 CARIGNAN NOIR 18 GRENACHE NOIR 19 LLADONER PELUT 20 MOURVEDRE 20 SYRAH 21 WINE PRODUCTION 23 THE SECRET ALCHEMY OF THE VINS DOUX NATURELS 23 FROM LEGEND TO HISTORY 23 THE MYSTERIES OF MUTAGE 23 WITH TIME, A UNIQUE BOUQUET 24 THE AOP DRY WINES AND THE IGP 24 WINE MAKING TECHNIQUES ADAPTED TO THE TERROIRS AND VARIETALS 24 - 2 - For free use. 14 APPELLATIONS D’ORIGINE CONTROLEE 26 AOP VINS DOUX NATURELS 26 AOP RIVESALTES 26 AOP MUSCAT DE RIVESALTES 28 AOP MAURY DOUX 28 AOP BANYULS 29 AOP BANYULS GRAND CRU 30 AOP DRY WINES 30 AOP COTES DU ROUSSILLON 30 AOP COTES DU ROUSSILLON LES ASPRES : 31 AOP COTES DU ROUSSILLON VILLAGES 31 AOP MAURY SEC 32 AOP COLLIOURE 32 IGP CÔTES CATALANES AND CÔTE VERMEILLE 33 APPENDIX 1: DISHES AND THE WINES THAT COMPLEMENT THEM 35 APPENDIX 2: SPECIFICATIONS 37 APPENDIX 3 : 2014 HARVEST SUMMARY 52 APPENDIX 4 : OVERVIEW OF SALES 55 CONTACTS 57 - 3 - For free use. -

Download Abstracts
JUNE 17–20, 2019 Napa Valley Marriott Hotel Technical Abstracts Napa, California USA 70 YEARS th NATIONAL Science: A Platform 70 for Progress CONFERENCE ASEV AMERICAN SOCIETY FOR ENOLOGY AND VITICULTURE 70 Technical Abstracts YEARS Oral Presentation Abstracts Wednesday, June 19 Enology—Phenolic Extraction ......................................................................................................................48–50 Viticulture—Impact of Red Blotch on Grape and Wine Composition ............................................ 51–54 Science: A Platform A Platform Science: Progress for Enology—Microbiology of Wine .................................................................................................................. 54–56 Viticulture—Managing Pests and Weeds ..................................................................................................57–59 Enology—Wine Chemistry: Oxidations and Aging .................................................................................59-61 Viticulture—Fruit Composition and Yield ..................................................................................................61-63 Thursday, June 20 Enology—Wine Macromolecules ........................................................................................................................ 64 Viticulture—Crop Load Management .........................................................................................................65-66 Enology—Wine Stability ................................................................................................................................ -

Simultaneous Determination of Arsenic, Manganese and Selenium in Human Serum by Neutron Activation Analysis
View metadata,Downloaded citation and from similar orbit.dtu.dk papers on:at core.ac.uk Dec 20, 2017 brought to you by CORE provided by Online Research Database In Technology Simultaneous determination of arsenic, manganese and selenium in human serum by neutron activation analysis Damsgaard, E.; Heydorn, Kaj; Larsen, N.A.; Nielsen, B. Publication date: 1973 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Damsgaard, E., Heydorn, K., Larsen, N. A., & Nielsen, B. (1973). Simultaneous determination of arsenic, manganese and selenium in human serum by neutron activation analysis. (Denmark. Forskningscenter Risoe. Risoe-R; No. 271). General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Risø Report No. 271 O Z 8o* Danish Atomic Energy Commission Bh Research Establishment Risø Simultaneous Determination of Arsenic, Manganese and Selenium in Human Serum by Neutron Activation Analysis by E. -
0M Mm EC Vc WM W
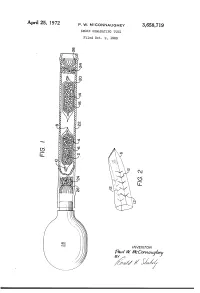
APII'I'l 25, 1972 P. w. MCCONNAUGHEY 3,658,719 SMOKE GENE-RATING TUBE Filed Oct. 9, 1969 \\\\\\\\\\~\ NJmyj \\\\\\\\\\\\\\\\\~\\\\\\\\\\\~Y \ R v.a la W. WMVc EC mm 0m (like), 1/17/; // - 1/ 1 .. 3,658,719 United States Patent 0 1C€ Patented Apr. 25, 1972 1 2 a perforated envelope 6 of polyethylene tubing heat 3,658,719 sealed at one end 8 and folded over at the other end 10. SMOKE GENERATING TUBE Paul W. McConnaughey, Wilkinsburg, Pa., assignor to As best seen in FIG. 2, the envelope has a plurality of Mine Safety Appliances Company, Pittsburgh, Pa. perforations 12 made as by cutting slits in the tubing Filed Oct. 9, 1969, Ser. No. 865,039 Wall. The perforations provide only small dimension Int. Cl. B01d; B01f; B01j 13/00 openings so that transfer of the volatile acid portion of US. Cl. 252—-359 A 2 Claims the reagent is substantially by diffusion; that is, there is no signi?cant convection ?ow of gases through the en velope. A great variety of methods of making suitable ABSTRACT OF THE DISCLOSURE 10 perforations are well known, such as, for example, slit ting or puncturing with needles or electrical sparks. Base A solid acid reagent and a solid base reagent are sepa reagent 14 is likewise contained in a breakable ampoule rately contained in a breakable ampoule that is enclosed 16 enclosed in perforated envelope 20. Both envelopes in a perforated envelope, which is in turn contained in are contained in a pliable tube 22 with suitable porous a pliable tube. -

An Overview on Botrytized Wines Revisão: Vinhos Botritizados
Ciência Téc. Vitiv. 35(2) 76-106. 2020 AN OVERVIEW ON BOTRYTIZED WINES REVISÃO: VINHOS BOTRITIZADOS Georgios Kallitsounakis1, Sofia Catarino1,2* 1LEAF (Linking Landscape Environment Agriculture and Food) Research Center, Instituto Superior de Agronomia, Universidade de Lisboa, Tapada da Ajuda, 1349-017 Lisboa, Portugal. 2CeFEMA (Centre of Physics and Engineering of Advanced Materials) Research Center, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais, 1, 1049-001 Lisboa, Portugal. * Corresponding author: Tel.: +351 21 3653246, e-mail: [email protected] (Received 08.06.2020. Accepted 29.08.2020) SUMMARY Noble rot wine is a specific type of sweet wine that derives from the infection of grape berries by a fungus called Botrytis cinerea. These wines are produced in specific wine regions around the world, with Sauternes region of France and Tokay region of Hungary being the most famous ones. The purpose of the current article is to provide a systematic review on the different stages of botrytized wines production, including a detailed analysis of the technical aspects involved. Specifically, it describes the process and development of berry infection by B. cinerea, and special emphasis is given to the main stages and operations of winemaking, conservation, aging and stabilization. A complex combination of a number of parameters (e.g., very specific environmental conditions) explains the rarity of noble rot occurrence and highlights the uniqueness of botrytized wines. RESUMO Os vinhos botritizados representam uma categoria específica de vinhos doces, sendo obtidos a partir de bagos de uva infectados pelo fungo Botrytis cinerea, através de um processo designado por podridão nobre. Estes vinhos são produzidos em regiões específicas do mundo, sendo Sauternes e Tokay, originários de França e Hungria respectivamente, os exemplos mais conhecidos a nível mundial. -

Submission of Evidence Guidelines-101508-Print
GUIDELINES FOR THE COLLECTION AND SUBMISSION OF FORENSIC EVIDENCE Delaware Department of Health and Social Services Office of the Chief Medical Examiner Forensic Sciences Laboratory 200 South Adams St., Wilmington, DE 19801 (302)-577-3420 © Copyright 2008 Richard T. Callery, M.D., F.C.A.P. , Chief Medical Examiner and Director of the Office of the Chief Medical Examiner Forensic Sciences Laboratory Mission Statement The OCME evidentiary guidelines are dedicated to all past, present, and future public servants who dedicate their careers to providing the state of Delaware with the highest degree of law enforcement, forensic science, and medical-legal death investigation services while maintaining the traditions of fairness, professionalism, and integrity. Delaware OCME - Forensic Sciences Laboratory Evidence Submission Guidelines 2008 Rev (0).pub — Page 3 — TABLE OF CONTENTS Introduction············································································································ 7 Using the Laboratory in the Judicial Process···························································· 8 Crime Scene Processing························································································· 9 General Submission Instructions ·······································································11-15 General Information·························································································· 11 Choosing Containers ························································································ -

Copyrighted Material
1 Water and Ethanol 1.1 Introduction From a macroscopic perspective, wine is a mildly acidic hydroethanolic solution. As shown in Table 1.1, water and ethanol represent ~97% w/w of dry table wines. Ethanol is the major bioactive compound in wine and its presence renders wine and other alcoholic beverages inhospitable to microbial pathogens. Understanding the physiochemical properties of wine will first require a review of the basic properties of water and water–ethanol mixtures. More thorough discussions of the unique properties of water, including those specific to the food chemistry, can be found elsewhere [1]. 1.2 Chemical and physical properties of water Water is a hydride of oxygen, but has unique properties compared to other hydrides of elements nearby on the periodic table, as shown in Table 1.2. For example, the boiling point of water (100 °C) is far above that of hydrides of adjacent elements on the periodic table: HF (19.5 °C), H2S (–60 °C), and NH3 (–33 °C). Thus, water exists as a liquid at room temperature, while the other hydrides exist as gases. Similarly, water also has a higher heat of vaporization, heat capacity, and freezing point than would be expected as compared to nearby hydrides. The unique properties of water are largely due to its ability to engage in intermolecular hydrogen (H) bond- ing, which results in stronger molecule‐to‐molecule interactions than in related compounds. ●● Oxygen is more electronegativeCOPYRIGHTED than hydrogen and an O–H MATERIALbond is more polarized than N–H or S–H. ●● The geometry and symmetry of an H2O molecule allows for four concurrent H bonds per water molecule. -

Vermouth Winemaking by Werner Roesener
Vermouth Winemaking by Werner Roesener The Vermouth wines described here are classified as sweet aperitif wines and are similar to the commercial products of sweet Cinzano or Martini. They are served chilled at 7 to 10 degrees Celsius as appetite stimulant before meals. They contain 17 to 19 percent alcohol and 7 to 9 percent sugar. Their particular flavour is derived from herbs. As an overview, the production involves making a suitable fortified base wine and then infusing herbs into it. To make a fortified base wine, the amateur winemaker has several options: 1. Adding alcohol to an existing table wine of typically 12 percent alcohol content This requires mixing 16.8 L of wine with 3.2 L of 40% alcohol or Vodka and 1.6 kg sugar to make a 20L batch. White table wine worksbest. Red wine can also be used, but very tannic wine should be avoided, becauseit may take several years of ageing to become drinkable. 2. Making a wine from start specifically for this purpose from grape juice or concentrate: The starting gravity should be adjusted with sugar or concentrate to 1100. A yeast with high alcohol tolerance must be used, i.e. Lalvin EC-1118 or sherry yeast. When fermentation is nearly complete as evident by reduced activity, adding small amounts of sugar (one cup per 20L batch) every few days will keep the fermentation going until activity stops, the wine will then contain about 16 to 18 percent alcohol. 3. Freeze concentrating table wine: A table wine containing about 12% alcohol is placed in a semi- soft container into a freezer and left to freeze solid for 48 hours. -

Brewing Glossary and Terms
Brewing Glossary and Terms Brewing Glossary Updated: May 6th 2020 Asian Beer Network Authored by: Neil Playfoot AsianBeerNetwork.com 1 Brewing Glossary and Terms Introduction Brewing Glossary I decided to put together a brewing glossary to help people with brewing terminology. As brewing evolves so does the terminology with new processes and practices developed. This brewing glossary will attempt to keep up to date with latest trends and brewing vocabulary. If you would like something added then please feel free to contact me. I have also added some terms mostly used in homebrewing as well to make the glossary as inclusive as possible. “Thanks for downloading this glossary; we hope it will be a valuable resource for you” This glossary used several sources which I will list at the end of this document. I have tried to list terms used universally in the brewing industry but appreciate that some terms maybe colloquial (for which I apologize). To contact me please email me at: [email protected] Have a good day and happy brewing! Cheers Neil AsianBeerNetwork.com 2 Brewing Glossary and Terms # 18TH AMENDEMENT: The 18th amendment of the United States Constitution effectively established the prohibition of alcoholic beverages in the United States by declaring illegal the production, transport and sale of alcohol (though not the consumption or private possession). 21ST AMENDEMENT: The 21st amendment to the United States Constitution repealed the 18th Amendment to the United States Constitution, which had mandated nationwide Prohibition on alcohol on January 17, 1920. A A.A.U: (Alpha acid units) The measurement, in percentage of alpha acid, of the potential bitterness in hops.