Effects of Azinphos-Methyl on Cholinergic Responses and General Health in Zebra Finches (Taeniopygia Guttata) After Previous Treatment with P,P¢-DDE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Insecticide Use for Vector-Borne Disease Control
WHO/CDS/NTD/WHOPES/GCDPP/2007.2 GLOBAL INSECTICIDE USE FOR VECTOR-BORNE DISEASE CONTROL M. Zaim & P. Jambulingam DEPARTMENT OF CONTROL OF NEGLECTED TROPICAL DISEASES (NTD) WHO PESTICIDE EVALUATION SCHEME (WHOPES) First edition, 2002 Second edition, 2004 Third edition, 2007 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication. CONTENTS Page Acknowledgements i Introduction 1 Collection of information 2 Data analysis and observations on reporting 3 All uses in vector control 6 Malaria vector control 22 Dengue vector control 38 Chagas disease vector control 48 Leishmaniasis vector control 52 Other vector-borne disease control 56 Selected insecticides – DDT 58 Selected insecticides – Insect growth regulators 60 Selected insecticides – Bacterial larvicides 62 Country examples 64 Annex 1. -

162998571.Pdf
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Liverpool Repository Please do not adjust margins Investigating the breakdown of the nerve agent simulant methyl paraoxon and chemical warfare agents GB and VX using nitrogen containing bases Received 00th January 20xx, Accepted 00th January 20xx Craig Wilson,a Nicholas J. Cooper,b Michael E. Briggs,a Andrew I. Cooper,*a and Dave J. Adams*c DOI: 10.1039/x0xx00000x A range of nitrogen containing bases was tested for the hydrolysis of a nerve agent simulant, methyl paraoxon (MP), and www.rsc.org/ the chemical warfare agents, GB and VX. The product distribution was found to be highly dependant on the basicity of the base and the quantity of water used for the hydrolysis. This study is important in the design of decontamination technology, which often involve mimics of CWAs. production of EA-2192 Introduction (S-(2-diisopropylaminoethyl) methylphosphonothioic acid), which exhibits roughly the same toxicity as VX itself.12 The Chemical warfare agents (CWAs) have a devastating effect on blister agent HD (Fig. 1d) can also undergo deactivation via the body and will disable or kill on exposure. Blister agents, such hydrolysis.13 However, its poor water solubility reduces the as sulfur mustard (HD, bis(2-chloroethyl) sulfide), target the skin efficiency of this decontamination method.14 As a result, and respiratory system causing severe pain and damage to the oxidation to the sulfoxide, or the addition of a co-solvent is most body whereas nerve agents, such as sarin (GB, isopropyl commonly used for HD deactivation.15-17 methylphosphonofluoridate) and VX (O-ethyl The high toxicity of CWAs means that research into their S-[2-(diisopropylamino)ethyl] methylphosphonothioate), target deactivation is often carried out using simulants. -

Past Use of Chlordane, Dieldrin, And
The Hazard Evaluation and Emergency Response Office (HEER Office) is part of the Hawai‘i Department of Health (HDOH) Environmental Health Administration, whose mission is to protect human health and the environment. The HEER Office provides leadership, support, and partnership in preventing, planning for, responding to, and enforcing environmental laws relating to releases or threats of releases of hazardous substances. Past Use of Chlordane, Dieldrin, and other Organochlorine Pesticides for Termite Control in Hawai‘i: Safe Management Practices around Treated Foundations or during Building Demolition This fact sheet provides building owners, demolition and construction contractors, developers, realtors, and others with an overview of the potential environmental concerns associated with the past use of organochlorine termiticides (pesticides used to control termites) in Hawai‘i. In addition, this fact sheet discusses methods for reducing exposure to organochlorine termiticides during building demolition or around the foundations of treated buildings and identifies resources for further information. What are organochlorine termiticides? Organochlorine termiticides are a group of pesticides that were used for termite control in and around wooden buildings and homes from the mid-1940s to the late 1980s. These organochlorine pesticides included chlordane, aldrin, dieldrin, heptachlor, and dichlorodiphenyltrichloroethane (DDT). They were used primarily by pest control operators in Hawaii’s urban areas, but also by homeowners, the military, the state, and counties to protect buildings against termite damage. In the 1970s and 1980s, the U.S. Environmental Protection Agency (EPA) banned all uses of these organochlorine pesticides except for heptachlor, which can be used today only for control of fire ants in underground power transformers. -
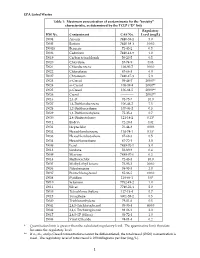
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level. -

Full Page Fax Print
RESISTANCE TO MALATHION, PIRIMIPHOS-METHYL AND FENITROTHION IN COLEOPTERA FROM STORED GRAINS. Ivania A. PACHECO; M. Regina SARTORI; Scheilla BOLONHEZI Instituto de Tecnologia de Alimentos Avenida Brasil, 2880 - Caixa Postal 139 Campinas - Sao Paulo - Brasil - CEP 13073 SUMMARY The objectives of this paper were:l) to verify the occurrance of resistance to malathion, pirimiphos-methyl and fenitrothion in populations of coleoptera from stored grains and 2) to obtain data that could contribute to the adequate control of these insects during storage, thus reducing losses. Populations of Sitophilus oryzae, ~. zeamais, Tribolium castaneum and Rhyzopertha dominica were collected from storage facilities located in different regions of Brazil, and tested for resistance to the tree insecticides according to the FAO Standard Method (FAO, 1975). Insects were collected in the States of Sao Paulo, Rio Grande do SuI, Santa Catarina, Goias, Acre and Rondonia. Twenty populations of S. oryzae, ten of S. zeamais, twenty of R. dominica and twenty -five of T. castan~ad already been tested for - resistance to the three insecticides. Seven populations of S. oryzae, six of R. dominica and ten of S. zean~is were susceptible 'to the th~ee insecticides. Resistance to malathion was showed in thirteen populations of S. oryzae, fourteen of ~. dominica and twenty-five of !. castaneum. Simultaneous cross-resistance to pirimiphos-methyl and fenitrothion was indicated in one population of ~. oryzae and eight of !. castaneum. Cross resistance only to pirimiphos-methyl was indicated in two populations of ~. oryzae, one of ~. dominica and one of !. castaneum and only to fenitrothion in two of R. dominica and two of T. -

Health Consultation Chevron Chemical Co
- • ! t Health Consultation Chevron Chemical Co. (Ortho Division) Orlando, Orange County, Florida CERCLIS NO. FLD004064242 June 1995 Prepared by Environmental Toxicology The Florida Department of Health and Rehabilitative Services Under a Cooperative Agreement With Agency for Toxic Substances and Disease Registry U.S. Public Health Service Department of Health and Human Services - ' ' Health Consultation - Chevron Chemical June 1995 Background and Statement of Issues The purpose of this health consultation is to interpret the results of indoor air monitoring for pesticides in two trailers adjacent to the Chevron Chemical Superfund site in Orlando, Florida. During a March 9, 1995 public meeting, two nearby residents expressed concerns that the insides of their trailers were contaminated with pesticides. We agreed to test their trailers for pesticide contamination. The Chevron Chemical Co. (Ortho Division) Superfund site is a former pesticide formulation plant and truck repair facility in Orlando, Florida (Figures 1-3, Appendix A). Past waste disposal practices contaminated soil and ground water. Stormwater run-off carried pesticide-contaminated soil to the adjacent Armstrong Trailer Park. In 1992, the Chevron Chemical Company removed the on-site contaminated soil. In 1994, they removed the contaminated soil from the Armstrong Trailer Park. In a 1995 public health assessment (ATSDR 1995), we found the site was a public health hazard because some residents of the adjacent Armstrong Trailer Park may have unknowingly eaten small amounts of soil contaminated with the pesticide chlordane. As a result, we estimated those residents have a moderately increased risk of liver cancer. Since Chevron cleaned up the chlordane-contaminated soil at this trailer park, we estimated the remaining cancer risk from chlordane is insignificant. -

Chronology of Pesticides Used on National Park Service Collections
Conserve O Gram June 2001 Number 2/16 Chronology Of Pesticides Used On National Park Service Collections The history of National Park Service pesticide use publication). Synonyms and trade names were policy for museum collection objects is obtained from the Merck Index, notes from the documented in various publications including IPM Coordinator, and two Internet sites Field Manual for Museums (Burns), Manual for (<http://chemfinder.com> and <http:// Museums (Lewis), versions of the Museum www.cdpr.ca.gov/cgi-bin/epa>). Handbook, Part I, and two versions of the Integrated Pest Management Information Manual. Not all of the chemicals listed in the Other non-policy sources include Coleman's accompanying charts were marketed as pesticides. Manual for Small Museums, object treatment Some are fungicides and microbiocides. One, reports and notes from NPS staff, and notes from Lexol, is a leather preservative and consolidant. the Office of the Integrated Pest Management All of these are included here because records (IPM) Coordinator. indicate they were applied to objects as pesticides. The two accompanying charts list the types of The potential for pesticide residue remaining on pesticides that may have been used on National collection objects is very high. Objects with such Park Service collections along with some common residues pose a health risk to curatorial staff and synonyms and trade names. to the public who come into physical contact with them, unless proper precautions are taken. Dates shown in blue on the chart represent Additional information on health and safety issues published recommendations for the use of and protective measures can be found in the pesticides. -

Pesticides and Toxic Substances
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES MEMORANDUM DATE: July 31, 2006 SUBJECT: Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides FROM: Debra Edwards, Director Special Review and Reregistration Division Office of Pesticide Programs TO: Jim Jones, Director Office of Pesticide Programs As you know, EPA has completed its assessment of the cumulative risks from the organophosphate (OP) class of pesticides as required by the Food Quality Protection Act of 1996. In addition, the individual OPs have also been subject to review through the individual- chemical review process. The Agency’s review of individual OPs has resulted in the issuance of Interim Reregistration Eligibility Decisions (IREDs) for 22 OPs, interim Tolerance Reassessment and Risk Management Decisions (TREDs) for 8 OPs, and a Reregistration Eligibility Decision (RED) for one OP, malathion.1 These 31 OPs are listed in Appendix A. EPA has concluded, after completing its assessment of the cumulative risks associated with exposures to all of the OPs, that: (1) the pesticides covered by the IREDs that were pending the results of the OP cumulative assessment (listed in Attachment A) are indeed eligible for reregistration; and 1 Malathion is included in the OP cumulative assessment. However, the Agency has issued a RED for malathion, rather than an IRED, because the decision was signed on the same day as the completion of the OP cumulative assessment. -

Qsar Analysis of the Chemical Hydrolysis of Organophosphorus Pesticides in Natural Waters
QSAR ANALYSIS OF THE CHEMICAL HYDROLYSIS OF ORGANOPHOSPHORUS PESTICIDES IN NATURAL WATERS. by Kenneth K. Tanji Principal Investigator and Jonathan 1. Sullivan Graduate Research Assistant Department of Land, Air and Water Resources University of California, Davis Technical Completion Report Project Number W-843 August, 1995 University of California Water Resource Center The research leading to this report was supported by the University of California Water Resource Center as part of Water Resource Center Project W-843. Table of Contents Page Abstract 2 Problem and Research Objectives 3 Introduction 5 Theoretical Background 6 QSAR Methodology 7 Molecular Connectivity Theory 8 Organophosphorus Pesticides 12 Experimental Determination of Rates 15 Results and Discussion 17 Principal Findings and Significance 19 References 34 List of Tables Page Table 1. Statistical relationship between OP pesticides and first-order MC/'s. 30 Table 2. Inherent conditions of waters used in experimental work. 16 Table 3. Estimated half-lives for organophosphorus esters derived from model. 31 Table 4. Half-lives and first-order MCI' sfor model calibration data set. 31 Table 5. Experimental kinetic data for validation set compounds, Sacramento. 33 List of Figures Page Figure 1. Essential Features OfQSAR Modeling Methodology. 21 Figure 2. Regression plot for In hydrolysis rate vs. 1st order MCl' s. 22 Figure 3. a 3-D molecular model, a line-segment model and a graphical model. 23 Figure 4. Molecular connectivity index suborders. 24 Figure 5. Chlorpyrifos and its fourteen fourth order path/cluster fragments. 25 Figure 6. Abridged MClndex output. 26 Figure 7. Parent acids of most common organophosphorus pesticides. 12 Figure 8. -

Environmental Health Criteria 63 ORGANOPHOSPHORUS
Environmental Health Criteria 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION Please note that the layout and pagination of this web version are not identical with the printed version. Organophophorus insecticides: a general introduction (EHC 63, 1986) INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY ENVIRONMENTAL HEALTH CRITERIA 63 ORGANOPHOSPHORUS INSECTICIDES: A GENERAL INTRODUCTION This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization World Health Orgnization Geneva, 1986 The International Programme on Chemical Safety (IPCS) is a joint venture of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization. The main objective of the IPCS is to carry out and disseminate evaluations of the effects of chemicals on human health and the quality of the environment. Supporting activities include the development of epidemiological, experimental laboratory, and risk-assessment methods that could produce internationally comparable results, and the development of manpower in the field of toxicology. Other activities carried out by the IPCS include the development of know-how for coping with chemical accidents, coordination -

Chlordane and Toxaphene Residues Following Cooking of Treated Channel Catfish Fillets
763 Journal of Food Protection, Vol. 63, No. 6, 2000, Pages 763±767 Copyright Q, International Association for Food Protection Chlordane and Toxaphene Residues following Cooking of Treated Channel Cat®sh Fillets C. R. SANTERRE,1* R. INGRAM,2 D. H. XU,3 G. W. LEWIS,4 AND L. G. LANE2 1Department of Foods and Nutrition, Purdue University, West Lafayette, Indiana 47907-1264; 2Mississippi State Chemical Laboratory, Mississippi State, Mississippi 39762; 3Department of Fisheries and Allied Aquacultures, Auburn University, Auburn, Alabama 36849; and 4Warnell School of Forest Resources, University of Georgia, Athens, Georgia 30602, USA MS 99-215: Received 28 July 1999/Accepted 17 December 1999 Downloaded from http://meridian.allenpress.com/jfp/article-pdf/63/6/763/1673844/0362-028x-63_6_763.pdf by guest on 27 September 2021 ABSTRACT The reduction in residues of chlordane and toxaphene following cooking (frying, baking, and smoking) of ®llets obtained from treated Channel cat®sh (Ictalurus punctatus) was determined. On average, cooking reduced moisture content by 17% and increased fat content by 28 to 274%. Frying reduced chlordane residues by 56 to 86% on a dry basis (db) or 84 to 92% on a percent fat basis (fb) when raw ®llets were compared to cooked ®llets. Baking and smoking reduced chlordane signi®cantly less (P , 0.05) than frying with reductions in residues of 12% and 9% (db) or 30% and 33% (fb), respectively. Frying reduced toxaphene residues by 40 to 49% (db) or 65 to 77% (fb), while baking and smoking reduced toxaphene by 35% and 24% (db) or 51% and 59% (fb), respectively. -

TOXICOLOGICAL PROFILE for CHLORDANE U.S. DEPARTMENT of HEALTH and HUMAN SERVICES Public Health Service Agency for Toxic Subs
TOXICOLOGICAL PROFILE FOR CHLORDANE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry May 1994 CHLORDANE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. CHLORDANE iii UPDATE STATEMENT A Toxicological Profile for Chlordane was released on December 1989. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary, but no less than once every three years. For information regarding the update status of previously released profiles contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, E-29 Atlanta, Georgia 30333 CHLORDANE vii CONTRIBUTORS CHEMICAL MANAGERS(S)/AUTHOR(S): Henry G. Abadin, M.S.P.H. ATSDR, Division of Toxicology, Atlanta, GA Ronald Baynes, D.V.M., M.S. Paul F. Goetchius, D.V.M. Syracuse Research Corporation, Syracuse, NY THE PROFILE HAS UNDERGONE THE FOLLOWING ATSDR INTERNAL REVIEWS: 1 . Green Border Review. Green Border review assures the consistency with ATSDR policy. 2 . Health Effects Review. The Health Effects Review Committee examines the health effects chapter of each profile for consistency and accuracy in interpreting health effects and classifying endpoints. 3 . Minimal Risk Level Review. The Minimal Risk Level Workgroup considers issues relevant to substance-specific minimal risk levels (MRLs), reviews the health effects database of each profile, and makes recommendations for derivation of MRLs. 4 . Quality Assurance Review. The Quality Assurance Branch assures that consistency across profiles is maintained, identifies any significant problems in format or content, and establishes that Guidance has been followed.