Appendix Common, Trade, and Chemical Names of Pesticides Mentioned in the Present Volume
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Insecticide Use for Vector-Borne Disease Control
WHO/CDS/NTD/WHOPES/GCDPP/2007.2 GLOBAL INSECTICIDE USE FOR VECTOR-BORNE DISEASE CONTROL M. Zaim & P. Jambulingam DEPARTMENT OF CONTROL OF NEGLECTED TROPICAL DISEASES (NTD) WHO PESTICIDE EVALUATION SCHEME (WHOPES) First edition, 2002 Second edition, 2004 Third edition, 2007 © World Health Organization 2007 All rights reserved. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either express or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. The named authors alone are responsible for the views expressed in this publication. CONTENTS Page Acknowledgements i Introduction 1 Collection of information 2 Data analysis and observations on reporting 3 All uses in vector control 6 Malaria vector control 22 Dengue vector control 38 Chagas disease vector control 48 Leishmaniasis vector control 52 Other vector-borne disease control 56 Selected insecticides – DDT 58 Selected insecticides – Insect growth regulators 60 Selected insecticides – Bacterial larvicides 62 Country examples 64 Annex 1. -
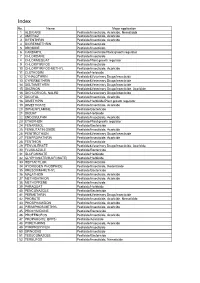
No. Name Major Application 1 ALDICARB Pesticide/Insecticide
Index No. Name Major application 1 ALDICARB Pesticide/Insecticide, Acaricide, Nematicide 2 AMITRAZ Pesticide/Insecticide, Acaricide 3 BIFENTHRIN Pesticide/Insecticide, Acaricide 4 BIORESMETHRIN Pesticide/Insecticide 5 BROMIDE Pesticide/Insecticide 6 CARBARYL Pesticide/Insecticide/Plant growth regulator 7 CHLORDANE Pesticide/Insecticide 8 CHLORMEQUAT Pesticide/Plant growth regulator 9 CHLORPYRIFOS Pesticide/Insecticide 10 CHLORPYRIFOS-METHYL Pesticide/Insecticide, Acaricide 11 CLETHODIM Pesticide/Herbicide 12 CYHALOTHRIN Pesticide&Veterinary Drugs/Insecticide 13 CYPERMETHRIN Pesticide&Veterinary Drugs/Insecticide 14 DELTAMETHRIN Pesticide&Veterinary Drugs/Insecticide 15 DIAZINON Pesticide&Veterinary Drugs/Insecticide, Acaricide 16 DICHLORVOS、NALED Pesticide&Veterinary Drugs/Insecticide 17 DICOFOL Pesticide/Insecticide, Acaricide 18 DIMETHIPIN Pesticide/Herbicide/Plant growth regulator 19 DIMETHOATE Pesticide/Insecticide, Acaricide 20 DIPHENYLAMINE Pesticide/Bactericide 21 DIQUAT Pesticide/Herbicide 22 ENDOSULFAN Pesticide/Insecticide, Acaricide 23 ETHEPHON Pesticide/Plant growth regulator 24 FENARIMOL Pesticide/Bactericide 25 FENBUTATIN OXIDE Pesticide/Insecticide, Acaricide 26 FENITROTHION Pesticide&Veterinary Drugs/Insecticide 27 FENPROPATHRIN Pesticide/Insecticide, Acaricide 28 FENTHION Pesticide/Insecticide 29 FENVALERATE Pesticide&Veterinary Drugs/Insecticide, Acaricide 30 FLUSILAZOLE Pesticide/Bactericide 31 GLUFOSINATE Pesticide/Herbicide 32 GLYPHOSATE(SULFOSATE) Pesticide/Herbicide 33 HEPTACHLOR Pesticide/Insecticide 34 HYDROGEN -

Propoxur United States Environmental Protection Agency
United States Prevention, Pesticides EPA738-R-97-009 Environmental Protection And Toxic Substances August 1997 Agency (7508W) Reregistration Eligibility Decision (RED) PROPOXUR UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES CERTIFIED MAIL Dear Registrant: I am pleased to announce that the Environmental Protection Agency has completed its reregistration eligibility review and decisions on the pesticide chemical case propoxur. The enclosed Reregistration Eligibility Decision (RED) contains the Agency's evaluation of the data base of this chemical, its conclusions of the potential human health and environmental risks of the current product uses, and its decisions and conditions under which these uses and products will be eligible for reregistration. The RED includes the data and labeling requirements for products for reregistration. It may also include requirements for additional data (generic) on the active ingredient to confirm the risk assessments. To assist you with a proper response, read the enclosed document entitled "Summary of Instructions for Responding to the RED." This summary also refers to other enclosed documents which include further instructions. You must follow all instructions and submit complete and timely responses. The first set of required responses is due 90 days from the receipt of this letter. The second set of required responses is due 8 months from the date of receipt of this letter. Complete and timely responses will avoid the Agency taking the enforcement action of suspension against your products. If you have questions on the product specific data requirements or wish to meet with the Agency, please contact the Special Review and Reregistration Division representative Bonnie Adler (703) 308-8523. -

Toxicity of Pyrethorids Co-Administered with Sesame Oil Against Housefly Musca Domestica L
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY 1560–8530/2007/09–5–782–784 http://www.fspublishers.org Toxicity of Pyrethorids Co-administered with Sesame Oil against Housefly Musca domestica L. SOHAIL AHMED1 AND MUHAMMAD IRFANULLAH Department of Agri-Entomology, University of Agriculture, Faisalabad–38040, Pakistan 1Corresponding author’s e-mail: [email protected] ABSTRACT The susceptibility of a laboratory reared strain of Musca domestica L. to cypermethrin 10 EC, fenpropathrin 20 EC, fenvalerate 20 EC and lambda cyhalothrin 2.5 EC, at different ranges of concentrations (250 to 2500 ppm) of the formulated insecticides in acetone alone and in combination with sesame oil in 1:1 and 1:2 ratio of insecticide: sesame oil was investigated. These concentrations in a volume of 5 mL were added to 25 g of granulated sugar in a petridish. House flies were fed on the insecticide coated sugar for 48 h. Knockdown and mortality data were recorded after 1, 2, 4, 6, 8, 12, 24 and 48 h and subjected to probit analysis. KD50 values of cypermethrin, lambda-cyhalothrin, fenpropathrin and fenvalerate in 1:1 ratio with sesame oil were 4297, 17188, 2324 and 8487 ppm, respectively as compared to 1915, 15034, 2608 and 4005 ppm respectively when these insecticides were applied alone. Similar fashion was seen in context of LC50 values. The pyrethroid + sesame oil combination in two ratios does not show the synergism in M. domestica. Key Words: M. domestica; Pyrethroids; Synergist; Sesame oil INTRODUCTION conventional insecticides as well as against cotton aphid (Aphis gossypii Glover) (Moore, 2005). Sesamin, a lignan Housefly (Musca domestica L.) causes a serious threat occurring in sesame’s seed oil has been reported as synergist to human and livestock health by transmitting many insecticide, antisseptic, bactericide (Bedigian et al., 1985). -

Pesticide Safety & Pesticide Categories
Pesticide Safety & Pesticide Categories Janet Hurley, & Don Renchie Texas A&M AgriLife Extension Service School IPM What is a pesticide • Any substance or mixture of substances intended for preventing, destroying, repelling, or mitigating any pest. • Any substance or mixture of substances intended for use as a plant regulator, defoliant, or desiccant. • Any nitrogen stabilizer. • A product is likely to be a pesticide if the labeling or advertising: • Makes a claim to prevent, kill, destroy, mitigate, remove, repel or any other similar action against any pest. • Indirectly states or implies an action against a pest. • Draws a comparison to a pesticide. • Pictures a pest on the label. Not considered pesticides Drugs used to control the diseases of humans or animals, which are regulated by the FDA Fertilizers and soil nutrients Certain low-risk substances such as cedar chips, garlic and mint oil are exempted from regulation by EPA (requires license) • 25b classification requires no signal word (mostly food-safe compounds) Pest control devices (i.e., mousetraps) are not pesticides, but subject to labeling requirements There are many kinds of pesticides How insecticides work: Modes of action • Nervous system poisons • Acts on the nerve • Metabolic inhibitors • Affect ability of target to process food • Hormone mimics • Disrupt normal growth & reproduction • Physical poisons • Physically damage insect • Repellents & attractants • All products have been assigned to groups based on their mode of Mode of action: • i.e. pyrethroids are Group 3; Action Neonicotinoids are Group 4A, Spinosad is Group 5, Diamides Classification are Group 28 • Product labels include the number corresponding to the mode of action group. -

U.S. Geological Survey National Water-Quality Assessment Program
U.S. Geological Survey National Water-Quality Assessment Program Stream water-quality analytes Major ions and trace elementsschedule 998 (20 constituents) Pesticides schedule 2437 (229 compounds) Alkalinity 1H1,2,4Triazole Arsenic 2,3,3Trichloro2propene1sulfonic acid (TCPSA) Boron 2,4D Calcium 2(1Hydroxyethyl)6methylaniline Chloride 2[(2Ethyl6methylphenyl)amino]1propanol Fluoride 2AminoNisopropylbenzamide Iron 2Aminobenzimidazole Lithium 2Chloro2',6'diethylacetanilide 2Chloro4,6diaminostriazine {CAAT} Magnesium (Didealkylatrazine) pH 2Chloro4isopropylamino6aminostriazine Potassium 2Chloro6ethylamino4aminostriazine {CEAT} Total dissolved solids 2ChloroN(2ethyl6methylphenyl)acetamide Selenium 2Hydroxy4isopropylamino6aminostriazine 2Hydroxy4isopropylamino6ethylaminostriazin Silica e {OIET} Sodium 2Hydroxy6ethylamino4aminostriazine Specific conductance 2Isopropyl6methyl4pyrimidinol Strontium 3,4Dichlorophenylurea Sulfate 3Hydroxycarbofuran Turbidity 3Phenoxybenzoic acid Vanadium 4(Hydroxymethyl)pendimethalin 4Chlorobenzylmethyl sulfoxide Suspended sediment 4Hydroxy molinate 4Hydroxychlorothalonil Nutrientsschedule 2430 (18 constituents) 4Hydroxyhexazinone A Inorganic carbon, suspended Acephate Dissolved inorganic carbon Acetochlor ammonia + organic nitrogen (unfilteredKjeldahl) Acetochlor oxanilic acid ammonia + organic nitrogen (filteredKjeldahl) Acetochlor sulfonic acid Ammonia as N, filtered Acetochlor sulfynilacetic acid nitrite, filtered Alachlor -

Appendix H EPA Hazardous Waste Law
Appendix H EPA Hazardous Waste Law This Appendix is intended to give you background information on hazardous waste laws and how they apply to you. For most U.S. Environmental Protection Agency (EPA) requirements that apply to the University, the Safety Department maintains compliance through internal inspections, record keeping and proper disposal. In Wisconsin, the Department of Natural Resources (DNR) has adopted the EPA regulations, consequently EPA and DNR regulations are nearly identical. EPA defines This Appendix only deals with "hazardous waste" as defined by the EPA. hazardous waste as Legally, EPA defines hazardous waste as certain hazardous chemical waste. This hazardous chemical Appendix does not address other types of regulated laboratory wastes, such as waste; radioactive, infectious, biological, radioactive or sharps. Chapter 8 descibes disposal procedures infectious and biohazardous waste for animals. Chapter 9 describes disposal procedures for sharps and other waste that are regulated by can puncture tissue. Chapter 11 discusses Radiation and the Radiation Safety for other agencies. Radiation Workers provides guidelines for the disposal of radioactive waste. Procedures for medical waste are written by the UW Hospital Safety Officer. The Office of Biological Safety can provide guidance for the disposal of infectious and biological waste. EPA regulations focus on industrial waste streams. As a result, many laboratory chemical wastes are not regulated by EPA as hazardous chemical waste. However, many unregulated chemical wastes do merit special handling and disposal If a waste can be procedures. Thus, Chapter 7 and Appendix A of this Guide recommend disposal defined as: procedures for many unregulated wastes as if they were EPA hazardous waste. -

Notwendigkeit Der Testung Von Biozidprodukten Und Deren Eluaten
Environmental Research of the Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety Project number: (FKZ) 3713 64 417 Report number: [entered by the UBA library] Necessity of testing biocidal products and their eluates within the regulatory authorization pro- cess aiming for an adequate environmental as- sessment of mixtures – extending the database for wood preservative products by Anja Coors 1, Pia Vollmar 1, Frank Sacher 2 1 ECT Oekotoxikologie GmbH, Böttgerstraße 2 – 14, 65439 Flörsheim am Main, Ger- many 2 TZW: DVGW-Technologiezentrum Wasser, Karlsruher Straße 84, 76139 Karlsruhe, Germany On behalf of the German Environment Agency Completion date November 2016 Environmental Risk Assessment of Biocidal Products as Mixtures Abstract Biocidal products are formulated preparations that contain one or more active substances and addi- tives added to serve various functions. They thereby represent intentional mixtures of chemical sub- stances that may reach the environment in their initial or in a changed composition. The present pro- ject addressed three aspects in a mixture risk assessment of biocidal products, which is required dur- ing the regulatory authorisation. These aspects range from direct regulatory application (component- based aquatic risk assessment of products) to more science-oriented exploratory work (indication for synergistic interactions and prediction of mixture toxicity in terrestrial organisms). No indication for synergistic interaction was found for the effects of fungicides that inhibit -

For Methyl Parathion
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES MEMORANDUM DATE: July 31, 2006 SUBJECT: Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides FROM: Debra Edwards, Director Special Review and Reregistration Division Office of Pesticide Programs TO: Jim Jones, Director Office of Pesticide Programs As you know, EPA has completed its assessment of the cumulative risks from the organophosphate (OP) class of pesticides as required by the Food Quality Protection Act of 1996. In addition, the individual OPs have also been subject to review through the individual- chemical review process. The Agency’s review of individual OPs has resulted in the issuance of Interim Reregistration Eligibility Decisions (IREDs) for 22 OPs, interim Tolerance Reassessment and Risk Management Decisions (TREDs) for 8 OPs, and a Reregistration Eligibility Decision (RED) for one OP, malathion.1 These 31 OPs are listed in Appendix A. EPA has concluded, after completing its assessment of the cumulative risks associated with exposures to all of the OPs, that: (1) the pesticides covered by the IREDs that were pending the results of the OP cumulative assessment (listed in Attachment A) are indeed eligible for reregistration; and 1 Malathion is included in the OP cumulative assessment. However, the Agency has issued a RED for malathion, rather than an IRED, because the decision was signed on the same day as the completion of the OP cumulative assessment. -

Organophosphate Poisoning : a Review
120 Sinha and Sharma Med J Indones Organophosphate poisoning : A review Parmod K. Sinha, Ashok Sharma Abstrak Pestisida organofosfat digunakan secara luas di seluruh dunia. Keracunan oleh bahan ini merupakan masalah kesehatan masyarakat, terutama di negara berkembang. Zat neurotoksik organofosfat merupakan bahan yang dianggap mengancam dalam bidang militer dan terorisme. Mekanisme toksisitas bahan ini adalah dengan cara menghambat asetilkolinesterase yang mengakibatkan menumpuknya neurotransmitor asetilkolin dan terjadi rangsangan terus-menerus pada reseptor asetilkolin pada sistem saraf sentral maupun perifer. Selain krisis kolinergik, organofosfat dapat menimbulkan berbagai sindrom neurologis, baik akut maupun kronik. Sedangkan gejala peralihan ( intermediate) terjadi 1-4 hari setelah krisis kolinergik teratasi. Pengobatan standar terdiri dari reaktivasi asetilkolinesterase dengan antidot golongan oksim (prolidoksim, oksidoksime, HI-6 dan HLo7), dan pengendalian efek biokimia asetilkolin dengan menggunakan atropin. Golongan oksim yang baru HI-6 dan Hlo7 merupakan reaktivator asetilkolinesterase yang lebih cocok dan efektif untuk keracunan akut dan berat dibandingkan dengan prolidoksim dan obidoksim. Penderita yang mendapat pengobatan segera, biasanya dapat sembuh dari toksisitas akut, namun gejala neurologis ikutan dapat saja terjadi. (Med J Indones 2003; 12: 120-6) Abstract Organophosphate pesticides are used extensively worldwide, and poisoning by these agents, particularly in developing nations is a public health problem. Organophosphorous -

162998571.Pdf
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of Liverpool Repository Please do not adjust margins Investigating the breakdown of the nerve agent simulant methyl paraoxon and chemical warfare agents GB and VX using nitrogen containing bases Received 00th January 20xx, Accepted 00th January 20xx Craig Wilson,a Nicholas J. Cooper,b Michael E. Briggs,a Andrew I. Cooper,*a and Dave J. Adams*c DOI: 10.1039/x0xx00000x A range of nitrogen containing bases was tested for the hydrolysis of a nerve agent simulant, methyl paraoxon (MP), and www.rsc.org/ the chemical warfare agents, GB and VX. The product distribution was found to be highly dependant on the basicity of the base and the quantity of water used for the hydrolysis. This study is important in the design of decontamination technology, which often involve mimics of CWAs. production of EA-2192 Introduction (S-(2-diisopropylaminoethyl) methylphosphonothioic acid), which exhibits roughly the same toxicity as VX itself.12 The Chemical warfare agents (CWAs) have a devastating effect on blister agent HD (Fig. 1d) can also undergo deactivation via the body and will disable or kill on exposure. Blister agents, such hydrolysis.13 However, its poor water solubility reduces the as sulfur mustard (HD, bis(2-chloroethyl) sulfide), target the skin efficiency of this decontamination method.14 As a result, and respiratory system causing severe pain and damage to the oxidation to the sulfoxide, or the addition of a co-solvent is most body whereas nerve agents, such as sarin (GB, isopropyl commonly used for HD deactivation.15-17 methylphosphonofluoridate) and VX (O-ethyl The high toxicity of CWAs means that research into their S-[2-(diisopropylamino)ethyl] methylphosphonothioate), target deactivation is often carried out using simulants. -

Full Page Fax Print
RESISTANCE TO MALATHION, PIRIMIPHOS-METHYL AND FENITROTHION IN COLEOPTERA FROM STORED GRAINS. Ivania A. PACHECO; M. Regina SARTORI; Scheilla BOLONHEZI Instituto de Tecnologia de Alimentos Avenida Brasil, 2880 - Caixa Postal 139 Campinas - Sao Paulo - Brasil - CEP 13073 SUMMARY The objectives of this paper were:l) to verify the occurrance of resistance to malathion, pirimiphos-methyl and fenitrothion in populations of coleoptera from stored grains and 2) to obtain data that could contribute to the adequate control of these insects during storage, thus reducing losses. Populations of Sitophilus oryzae, ~. zeamais, Tribolium castaneum and Rhyzopertha dominica were collected from storage facilities located in different regions of Brazil, and tested for resistance to the tree insecticides according to the FAO Standard Method (FAO, 1975). Insects were collected in the States of Sao Paulo, Rio Grande do SuI, Santa Catarina, Goias, Acre and Rondonia. Twenty populations of S. oryzae, ten of S. zeamais, twenty of R. dominica and twenty -five of T. castan~ad already been tested for - resistance to the three insecticides. Seven populations of S. oryzae, six of R. dominica and ten of S. zean~is were susceptible 'to the th~ee insecticides. Resistance to malathion was showed in thirteen populations of S. oryzae, fourteen of ~. dominica and twenty-five of !. castaneum. Simultaneous cross-resistance to pirimiphos-methyl and fenitrothion was indicated in one population of ~. oryzae and eight of !. castaneum. Cross resistance only to pirimiphos-methyl was indicated in two populations of ~. oryzae, one of ~. dominica and one of !. castaneum and only to fenitrothion in two of R. dominica and two of T.