Impact of Wind and River Flow on the Timing of the Rivers Inlet Spring Phytoplankton Bloom
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Technical Report No. 70
FISHERIES RESEARCH BOARD OF CANADA TECHNICAL REPORT NO. 70 1968 FISHERIES RESEARCH BOARD OF CANADA Technical Reports FRE Technical Reports are research documents that are of sufficient importance to be preserved, but which for some reason are not aopropriate for scientific pUblication. No restriction is 91aced on subject matter and the series should reflect the broad research interests of FRB. These Reports can be cited in pUblications, but care should be taken to indicate their manuscript status. Some of the material in these Reports will eventually aopear in scientific pUblication. Inquiries concerning any particular Report should be directed to the issuing FRS establishment which is indicated on the title page. FISHERIES RESEARCH BOARD DF CANADA TECHNICAL REPORT NO. 70 Some Oceanographic Features of the Waters of the Central British Columbia Coast by A.J. Dodimead and R.H. Herlinveaux FISHERIES RESEARCH BOARD OF CANADA Biological Station, Nanaimo, B. C. Paci fie Oceanographic Group July 1%6 OONInlTS Page I. INTHOOOCTION II. OCEANOGRAPHIC PlDGRAM, pooa;OORES AND FACILITIES I. Program and procedures, 1963 2. Program and procedures, 1964 2 3. Program and procedures, 1965 3 4 III. GENERAL CHARACICRISTICS OF THE REGION I. Physical characteristics (a) Burke Channel 4 (b) Dean Channel 4 (e) Fi sher Channel and Fitz Hugh Sound 5 2. Climatological features 5 (aJ PrectpitaUon 5 (b) Air temperature 5 (e) Winds 6 (d) Runoff 6 3. Tides 6 4. Oceanographic characteristics 7 7 (a) Burke and Labouchere Channels (i) Upper regime 8 8 (a) Salinity and temperature 8 (b) OJrrents 11 North Bentinck Arm 12 Junction of North and South Bentinck Arms 13 Labouchere Channel 14 (ii) Middle regime 14 (aJ Salinity and temperature (b) OJrrents 14 (iii) Lower regime 14 (aJ 15 Salinity and temperature 15 (bJ OJrrents 15 (bJ Fitz Hugh Sound 16 (a) Salinlty and temperature (bJ CUrrents 16 (e) Nalau Passage 17 (dJ Fi sher Channel 17 18 IV. -

British Columbia Regional Guide Cat
National Marine Weather Guide British Columbia Regional Guide Cat. No. En56-240/3-2015E-PDF 978-1-100-25953-6 Terms of Usage Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment Canada’s Inquiry Centre at 1-800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. Disclaimer: Her Majesty is not responsible for the accuracy or completeness of the information contained in the reproduced material. Her Majesty shall at all times be indemnified and held harmless against any and all claims whatsoever arising out of negligence or other fault in the use of the information contained in this publication or product. Photo credits Cover Left: Chris Gibbons Cover Center: Chris Gibbons Cover Right: Ed Goski Page I: Ed Goski Page II: top left - Chris Gibbons, top right - Matt MacDonald, bottom - André Besson Page VI: Chris Gibbons Page 1: Chris Gibbons Page 5: Lisa West Page 8: Matt MacDonald Page 13: André Besson Page 15: Chris Gibbons Page 42: Lisa West Page 49: Chris Gibbons Page 119: Lisa West Page 138: Matt MacDonald Page 142: Matt MacDonald Acknowledgments Without the works of Owen Lange, this chapter would not have been possible. -

Burke. Channel
~.. ~. HSHtRI tS atSu~R&' " . BGA'R~ ~f Cl~llA . .~ ~. - . ' ~ . B\OtOG\b~t ,Sl~l\Q~, .. , - '-. '.'· Sf. Jn1\tiJs.M£WfnU:Rnl~ND .., " ).0 '.;; I" . .' -... ... -.. - . IJI8:8 •••_$ _R_ . 8~~B~ (;P .·' BOA •• ," ' OF" CA -:N ' A:P&-~ " . :- ', ..~:ANJ1SCRIPT REP'O~T . SEItIES ,No. 976 .. .. " • j. _ ' - - . ' • I _I, ' . [ , '<" . ~ .. r, . Drift Card Beiea8e.8 ~ ~n, d .ReeQverielf . , .. , ... ..... • ' -' .0 >' In . '. , -,. 'Burke . Channel - .- \ . i j' , " >.- British ,Columbia, 1 1967 .. ,. 0 • b1 " ~"" 'It • ' .J 1t~1I~ ', Herlinveaq~ . .- , ~. :" . - , ", .BlQ1o.gical S-tat;on, NJlIi.tmo, B.C.; - .. i?~cifl~Qceanogr~phic Group ' . - , ' , ,~ ."~. March 1968 FISHERIES RE8EAR~B BOARD OF ~ANADA MANUSCRIPT REPORT SERIES No. 970 Drift Card Releases and Recoveries • In Burke Channel British Columbia, 1967 by R. H. Herlinveaux Biological Station, Nanaimo, B.C. Pacific Oceanographic Group March 1968 Programmed by The Canadian Committee on Oceanography Drift Card Releases and Recoveries in Burke Channel, British Columbi.a, 1967 by E..H. Herlinveaux INTRODUCTION Burke Channel is the main migratory route of the pink salmon moving into and out of the Bella Coola River system (Figure 1). On their way to the sea, the pink salmon spend approximately twq months (mid~April to mid-June) in the system, during which time a significant and variable mortality occurs. The mortality must be associated partly with environmental factors. among which water movements are considered to be most significant. Water movements may either directly tiansport the fish, or, by altering the salinity distribution, and! or by concentrating or dispersing food organisms, modify their travel. Furthermore , variation in water movements may change the nutrient supply, which in turn will alter production of food necessary for growth and survivaL An extensive 5=year study of the l!spring Qceanographic conditionsl! in the system was terminated in 1967. -
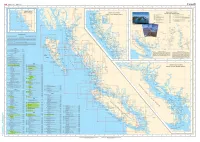
CHS Index Chart
Fisheries and Oceans Pi'lches et Oceans Canada Canada Canada ••• 137 ° 136 " 134" 133 ° 131° 129 ° ,.. 126 ° 125 " 124 " 123° 122° 119° 124 ° 118 ° GENERAL CHARTS CARTES GENERALES SMALL-CRAFT CHARTS REV ILLAG IGEDO LARGER SCALE CHARTS ISLAND CARTES POUR EMBARCATIONS CARTES A PLUS GRANDE ECHELLE 3050 Kootenay Lake and Rovet 75 000 3311 Sunshtno Coast- Vancouver Ha rbour lo/A 3052 Okanagan Lako so 000 Desolatoon Sound 40 000 3053 Shuswap Lake so 000 331:2 JerviS Intel ond/et Do•o latoon Sound 0 305S Waneta to /~ Hugh Keen leyside Dam 20 000 Vo,ous Scolo•JEche tle• vo"h• > z 3056 Hugh Koon loySido Dam to/A Burlon 40 000 3313 Gull Islands and Ad jacent Watotways/el les Vo1es Navigables Ad1acentes ~ ' 3057 Button IO/~ 1\rrowhood 40 000 Variou• Scale•/Echel le• vo"~"' 3058 Arrowhead lo/6 Rovo lotoko Go «m• 20 000 3488 Fro5er River/F I&uve Fraser, Cre•<ent l5land 3061 " "'"'on Lake and/ol Hamson R1ver '""'' to/~ Hon loon Mills 20 000 Harrison Lake 40 000 3469 Fraser Rovor/Fiouve Fr8set, Pattullo B"dgo Harrison R1ver 30 000 to/a Crescent Is land 20 000 Pitt River and/ot Poll l ak e 25 000 Stuaot L a~e (Not•howniP••rnd•qu o!) 50 000 54 " ~f--- ' ~ 0 "" I < ''"0' 't)Go iUn "?1- Cocoov• 3053 Foo ..o<o o l 0 "' GJ ,. Shu wap .•. Lake CANAOA !'; "'""""'' •·o~ d 130" 125° 120 " •5"omouo .,cocho Cceo> ,... ,. ' GJ ... ' <om l oops ~ DIXON E'N TRANCE' LEGENO/LEGENDE • •• • Scales smaller ttlan 1"40 000 Ectlellas plus petites qua 1:40 000 '' GJ Scales 1:40 000 and larger Ectlelles 1: 40 000 et plus grande& CHART SCALE Chart soale os the rat10 of one umt of d1stance 011 the cha" to the actual d.stance on the Earth's surface expressed on tho same unots. -
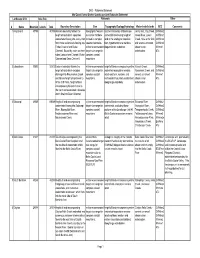
LU Boundary Rationale
DMC - Rationale Statement Mid Coast Forest District Landscape Unit Rationale Statement Landscape Unit Area (ha) Rationale Other # Name Mountain Islands Total Boundary Description Size Topography/Ecology/Hydrology Watersheds/Islands BEC Comments 1 King Island 40759 40759 Western boundary follows the topographic features southern boundary of landscape Jenny Inlet, Fog Creek, CWHms2 height of land which separates are similar to those unit established along height of Green River, Loken CWHvm1 watersheds flowing into Jenny Inlet located in complex land at the ecological transition Creek, Hole in the Wall, CWHvm2 from those watersheds flowing into coastal mountains- from hypermaritime to maritime and several unnamed CWHvm3 Fisher Channel and Burke within recommended biogeoclimatic subzones streams and MHmm1 Channel. Bound by water on three target size range for waterbodies ATc sides (Labouchere Channel, Burke complex coastal Channel and Dean Channel). mountains 2 Labouchere 50803 50803 Eastern boundary follows the within recommended height of land encompassing entire Nusash Creek, CWHms2 height of land which excludes target size range for watershed-ecosystem remains Nooseseck Creek, and CWHvm3 drainage into Nieumiamus Creek complex coastal intact-southern, western, and several unnamed MHmm1 and follows height of land west to mountains. northeastern boundary established streams and ATc White Cliff Point. Height of land along large waterbody waterbodies. encompassing Nusash Creek to the north and associated tributaries which flow into Dean Channel. 3 Saloompt 69049 69049 Height of land encompassing within recommended height of land encompassing entire Saloompt River, CWHds2 watersheds flowing into Saloompt target size range for watersheds, excluding those Noosgulch River, CWHms2 River, Noosgulch River, complex coastal portions within Landscape Unit #5 Tseapseahoolz Creek, CWHws2 Necleetsconnay River and mountains (Bella Coola)-ecosystem remains Talcheazoone Lakes, MHmm2 Nieumiamus Creek. -

In BC Less Than One Percent of the Total Marine Environment Provides Any Level of Protection for Species and Their Habitat
CONSERVING MARINE BIODIVERSITY In BC less than one percent of the total marine environment provides any level of protection for species and their habitat. he ecological balance in the sea is being threatened by human activities. Scientists point to overharvesting, pollution, habitat degradation, climate T change and the introduction of alien species as the most serious threats to marine biological diversity (or “biodiversity”). The signs of human impacts on the marine environment are becoming more “Because the destruction of our frequent and more serious. environment has proceeded so In Pacific Canada, as in other parts of the country and the world, we do not far, preventing things from have a history of sustainably managing living marine resources. The traditional vanishing is essential but not approach has been reactive—acting only after the damage is done, by closing sufficient. Rather than adopting fisheries or cleaning up sources of pollution. a goal of stopping the loss of We need a new approach that focuses on the conservation of marine biodiversity. endangered biological resources, the goal of marine This strategy should include: controlling sources of pollution; integrated coastal zone biodiversity conservation management; direct regulation of marine resources; community stewardship; use of should be to ensure that they economic incentives (or disincentives); and establishment of marine protected areas do not become endangered, (MPAs). that is, the goal should be to maintain the integrity of life.” Dr. Elliot Norse, Global Marine Biological Diversity Marine protected areas (MPAs) are a much-needed, but underutilized tool for conserving marine biodiversity. Cover: Standoff between predator and prey—red Irish lord sculpin and pygmy rock crab in Browning Pass. -

Hyp3 PROJECT Pattern, Process, and Productivity in Hypermaritime Forests of Coastal British Columbia 2005 a SYNTHESIS of 7-YEAR RESULTS
SPECIAL REPORT THE HyP3 PROJECT Pattern, Process, and Productivity in Hypermaritime Forests of Coastal British Columbia 2005 A SYNTHESIS OF 7-YEAR RESULTS Ministry of Forests Forest Science Program The HyP3 Project Pattern, Process, and Productivity in Hypermaritime Forests of Coastal British Columbia A Synthesis of 7-Year Results Compiled & Edited by: Allen Banner, Phil LePage, Jen Moran, & Adrian de Groot Ministry of Forests Forest Science Program The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the Government of British Columbia of any product or service to the exclusion of any others that may also be suitable. Contents of this report are presented as information only. Funding assistance does not imply endorse- ment of any statements or information contained herein by the Government of British Columbia. Library and Archives Canada Cataloguing in Publication Data Main entry under title: The HyP3 Project : pattern, process and productivity in hypermaritime forests of coastal British Columbia : a synthesis of 7-year results (Special report series, 0843-6452 ; 10) Includes bibliographical references: p. 0-7726-5320-8 1. Forest ecology - British Columbia - Pacific Coast. 2. Sustainable forestry - British Columbia - Pacific Coast. 3. Forest management - British Columbia - Pacific Coast. 4. Forests and forestry - British Columbia - Pacific Coast. I. Banner, Allen, 1954- . II. British Columbia. Forest Science Program. II Series: Special report series (British Columbia. Ministry of Forests) ; 10. 106.2.737 2005 333.75'09711 2005-960066-7 Citation: Banner, A., P. LePage, J. -

OCEANOGRAPHIC STUDY of the BURKE CHANNEL -ESTUARY
p.; / OCEANOGRAPHIC STUDY OF tHE BURKE CHANNEL -ESTUARY 1966-1967 R.H. Herlinveaux ENVIRONMENT CANADA Fisheries and Marine Service Marine Sciences Directorate Pacific Region 1230 Government St. Victoria, 8.C. MARINE SCIENCES DIRECTORATE , PACIFlC REGION PACIFIC MARINE SCIENCE REPORT OCEANOGRAPHIC STUDY OF THE BURKE CHANNEL ESTUARY: 1966-1967 by R.H. HerZinveaux Victoria , B.C. Marine Sciences Directo rate , Pacific Region Environment Canada Septembe r 1973 Table of Contents Introduction . 1 AreaS' Studied 2 Facilities . 3 Program and Procedures: 1966 3 Program and Procedures: 1967 . 5 Results: 1966 A. Wind , Temperature , Salinities 7 B. Time Series: Burke Narrows 12 · . C. Drift Measurements . 13 Results: 1967 A. Time Series . • . .' 16 B. General Distribution of Surface Oceanographic Properties . • 19 C. Surface Drift Me asurements . 20 . D. Drift Card Program . • . 20 Continuous Current Meter Observations 22 E. • • Winds F. 25 Discussion and Conclusions . • • 26 Appendix . I . Scientific Party . Ack now ledgements . References . Lis t Figures . of . OCEANOGRAPHIC STUDY OF THE BURKE CHANNEL ESTUARY: 1966-1967 by R. H. Herlinveaux INTRODUCTION Burke Ch anne l is located on the coast of British Columbia approximately 240 mi les northwest of Vancouver (Figure 1) . It is the main route through whi ch pink salmon migrate into and out of the Bella Coola River system from the Pacific Ocean . During the period (April, May, June) \vhen the young pink salmon are circulating in the Burke Ch annel system prior to entrainment into the sea , it is believed that a vari able and often significant mortality occurs. It has been surmised that this mortality rate is associate d primarily with the interplay between several environmental factors; water movements may be among the mo st important factors since they may direc tly transport th e fish or alter migratory routes by modifying the sa linity dis tribution . -

Code Search Results
ECAS Code List Code Table Code Value Description Where Used in Application Notes ADS_INSECT_SPECIES_CODE MPB Mountain Pine Beetle Interior UNK Unknown ADS_SPECIES_DAMAGE_CATGRY_CODE G Green Interior Expires on Dec 1, 2007 GA Green Attack RA Red Attack YA Gray Attack DP Dead Potential Expires on Dec 1, 2007 Ads_Location_Code CARV Campbell River Coast CHWK Chilliwack HOUS Houston MERR Merritt NANA Nanaimo PRRU Prince Rupert TERR Terrace VANC Vancouver VICT Victoria Appraisal_Amendment_Type_Code ADD Addition Coast DEL Deletion Appraisal_Category_Code Common N Initial ADS R Reappraisal D Redetermination Expires on Aug 1, 2013 P Post-Harvest ADS Effective on Apr 1, 2019 Apprsl_Certification_Type_Code R Reviewed Common S Supervised P Personally Prepared Appraisal_Culvert_Type_Code W Wooden Coast M Metal Coast T Tabular Interior Appraisal_Document_Type_Code BR Detailed Engineering - Bridge Repairs Coast Expired Dec 15, 2019 CAF Cruise - Cruise Analysis Form Coast CEF1 NDC Form #1 Coast CEF2 NDC Form #2 Coast CEF3 NDC Form #3 Coast CEF4 NDC Form #4 Coast CEF5 NDC Form #5 Coast CEF6 NDC Form #6 Coast CEF7 NDC Form #7 Coast CEF8 NDC Form #8 Coast CEF9 NDC Form #9 Coast CEF10 NDC Form #10 Coast CEF11 NDC Form #11 Coast CEF12 NDC Form #12 Coast CEF13 NDC Form #13 Coast CEF14 NDC Form #14 Coast CEF15 NDC Form #15 Coast CEF16 NDC Form #16 Coast CEF17 NDC Form #17 Coast CEF18 NDC Form #18 Coast CEF19 NDC Form #19 Coast CEF20 NDC Form #20 Coast SOFZ Specified Operations - Fibre Recovery Zone Coast SOMS Specified Operations - Miscellaneous Coast DCDA -

CANADA British Columbia Groundfish Fisheries and Their Investigations
CANADA British Columbia Groundfish Fisheries and Their Investigations in 2019 April 2020 Prepared for the Technical Sub-Committee of the Canada-United States Groundfish Committee Compiled by M. Cornthwaite, L. Granum, and D. Haggarty Fisheries and Oceans Canada Science Branch, Pacific Biological Station, Nanaimo, British Columbia V9T 6N7 i Table of Contents I. Agency Overview .................................................................................................................1 II. Surveys ................................................................................................................................3 A. Databases and Data Acquisition Software ....................................................................3 B. Commercial Fishery Monitoring and Biological Sampling ..............................................3 C. Research Surveys .........................................................................................................4 III. Reserves .............................................................................................................................9 IV. Review of Agency Groundfish Research, Assessment and Management ..........................10 A. Hagfish .......................................................................................................................10 B. Dogfish and other sharks ............................................................................................11 C. Skates .........................................................................................................................13 -

Chapter 4 Seasonal Weather and Local Effects
BC-E 11/12/05 11:28 PM Page 75 LAKP-British Columbia 75 Chapter 4 Seasonal Weather and Local Effects Introduction 10,000 FT 7000 FT 5000 FT 3000 FT 2000 FT 1500 FT 1000 FT WATSON LAKE 600 FT 300 FT DEASE LAKE 0 SEA LEVEL FORT NELSON WARE INGENIKA MASSET PRINCE RUPERT TERRACE SANDSPIT SMITHERS FORT ST JOHN MACKENZIE BELLA BELLA PRINCE GEORGE PORT HARDY PUNTZI MOUNTAIN WILLAMS LAKE VALEMOUNT CAMPBELL RIVER COMOX TOFINO KAMLOOPS GOLDEN LYTTON NANAIMO VERNON KELOWNA FAIRMONT VICTORIA PENTICTON CASTLEGAR CRANBROOK Map 4-1 - Topography of GFACN31 Domain This chapter is devoted to local weather hazards and effects observed in the GFACN31 area of responsibility. After extensive discussions with weather forecasters, FSS personnel, pilots and dispatchers, the most common and verifiable hazards are listed. BC-E 11/12/05 11:28 PM Page 76 76 CHAPTER FOUR Most weather hazards are described in symbols on the many maps along with a brief textual description located beneath it. In other cases, the weather phenomena are better described in words. Table 3 (page 74 and 207) provides a legend for the various symbols used throughout the local weather sections. South Coast 10,000 FT 7000 FT 5000 FT 3000 FT PORT HARDY 2000 FT 1500 FT 1000 FT 600 FT 300 FT 0 SEA LEVEL CAMPBELL RIVER COMOX PEMBERTON TOFINO VANCOUVER HOPE NANAIMO ABBOTSFORD VICTORIA Map 4-2 - South Coast For most of the year, the winds over the South Coast of BC are predominately from the southwest to west. During the summer, however, the Pacific High builds north- ward over the offshore waters altering the winds to more of a north to northwest flow. -

Fjords! (Especially the Kitimat Fjord System)
Bangarang February 2014 Backgrounder1 Fjords! (especially the Kitimat Fjord System) Eric Keen Abstract Fjords are awesome, and sometimes there are whales in them. This raises some questions: First of all, where do fjord babies come from? (Some Great Ice Cream Scoop in the Sky???) Why should we care about them? (Where to begin?) Where in the world do they happen? (A Goldilocks temperate zone and it’s perty too!) Why are British Columbia’s fjords the best? (If you’ve ever visited them you wouldn’t be asking such stupid questions.) What do you know about the fjords of the Gitga’at Territory and the Bangarang study area? (Ah, the Kitimat Fjord System! Where to begin?!) Contents Introduction Defined Worldwide British Columbia The Kitimat Fjord System Physical-Chemical Oceanography Freshwater Input Circulation Estuarine Wind-Driven Tidal Features Property Distribution Vertical Structure Horizontal Structure Seasonality Other Properties (pH, DO, nutrients) Deep & Bottom Waters Sediments Literature Cited 1 Bangarang Backgrounders are imperfect but rigorous reviews – written in haste, not peer-reviewed – in an effort to organize and memorize the key information for every aspect of the project. They will be updated regularly as new learnin’ is incorporated. 1 Introduction Due to the complexity of their ecological space, coastal waters comprise some of the most diverse and productive marine habitats on earth (Levin & Dayton 2009). This high biodiversity is the cornerstone of lucrative fisheries, tourism industries, and innumerable ecosystem services, including nutrient cycling, nursery habitat, food web support, carbon sequestration, and tourism revenue (Turner 2000). And yet, while the health of coastal waters is the most economically valuable and easily monitored of marine systems, they are also among the most endangered (Gray 1997).