Catalyzed Chemoselective Amide Reduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amide, and Paratoluenesulfonamide on the Amide of Silver,On the Imides
68 CHEMISTRY: E. C. FRANKLIN METALLIC SALTS OF AMMONO ACIDS By Edward C. Franklin DEPARTMENT OF CHEMISTRY, STANFORD UNIVERSITY Presented to the Academy, January 9. 1915 The Action of Liquid-Ammonia Solutions of Ammono Acids on Metallic Amides, Imides, and Nitrides. The acid amides and imides, and the metallic derivatives of the acid amides and imides are the acds, bases, and salts respectively of an ammonia system of acids, bases, and salts.1 Guided by the relationships implied in the above statement Franklin and Stafford were able to prepare potassium derivatives of a considerable number of acid amides by the action of potassium amide on certain acid amides in solution in liquid ammonia. That is to say, an ammono base, potassium amide, was found to react with ammono acids in liquid ammonia to form ammono salts just as the aquo base, potassium hydrox- ide, acts upon aquo acids in water solution to form aquo salts. Choos- ing, for example, benzamide and benzoic acid as representative acids of the two systems, the analogous reactions taking place respectively in liquid ammonia and water are represented by the equations: CH6CONH2+KNH2 = C6H5CONHK + NHs. CseHCONH2 + 2KNH2 = CIHsCONK2 + 2NH3. CH6tCOOH + KOH = CIH6COOK + H2O. The ammono acid, since it is dibasic, reacts with either one or two molecules of potassium amide to form an acid and a neutral salt. Having thus demonstrated the possibility of preparing ammono salts of potassium by the interaction of potassium amide and acid amides in liquid ammonia solution, it was further found that ammono salts of the heavy metals may be prepared by the action of liquid ammonia solutions of ammono acids on insoluble metallic amides, imides, and nitrides-that is, by reactions which are analogous to the formation of aquo salts in water by the action of potassium hydroxide on insoluble metallic hydroxides and oxides. -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -
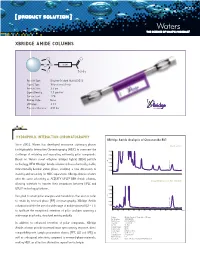
Xbridge Amide Columns
[ PRODUCT SOLUTION ] XBRIDGE AMIDE COLUMNS O O O Si Linker O NH 2 Particle Type: Ethylene Bridged Hybrid [BEH] Ligand Type: Trifunctional Amide Particle Size: 3.5 µm Ligand Density: 7.5 µmol/m2 Carbon Load: 12% Endcap Style: None pH Range: 2-11 Pressure Tolerance: 400 bar HYDROPHILIC INTERacTION CHROMATOGRAPHY XBridge Amide Analysis of Ginsenoside Rb1 Since 2003, Waters has developed innovative stationary phases Orento extract for Hydrophilic Interaction Chromatography [HILIC] to overcome the 0.010 challenge of retaining and separating extremely polar compounds. 0.008 Based on Waters novel ethylene bridged hybrid [BEH] particle 0.006 AU technology, NEW XBridge™ Amide columns utilize a chemically stable, 0.004 0.002 Ginsenoside Rb1 trifunctionally-bonded amide phase, enabling a new dimension in 0.000 stability and versatility for HILIC separations. XBridge Amide columns offer the same selectivity as ACQUITY UPLC® BEH Amide columns, 20 µg/mL Ginsenoside Rb1 standard allowing scientists to transfer their separations between HPLC and 0.010 ® UPLC technology platforms. 0.008 0.006 Designed to retain polar analytes and metabolites that are too polar AU 0.004 to retain by reversed-phase [RP] chromatography, XBridge Amide 0.002 columns facilitate the use of a wide range of mobile phase pH [2 – 11] 0.000 Ginsenoside Rb1 to facilitate the exceptional retention of polar analytes spanning a 0.00. 5 1.01. 5 2.02. 5 3.03. 5 4.04. 5 5.05. 5 6.06. 5 min wide range in polarity, structural moiety and pKa. Column: XBridge Amide, 3.5 µm, 4.6 x 150 mm Part Number: 186004869 Mobile Phase : 80/20 ACN/H2O In addition to enhanced retention of polar compounds, XBridge Flow Rate: 1.4 mL/min Inj. -

COMMUNICATION Catalytic Ester and Amide To
Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C−O and C−C Bond Activation Item Type Article Authors Yue, Huifeng; Guo, Lin; Liao, Hsuan-Hung; Cai, Yunfei; Zhu, Chen; Rueping, Magnus Citation Yue H, Guo L, Liao H-H, Cai Y, Zhu C, et al. (2017) Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C−O and C −C Bond Activation. Angewandte Chemie International Edition 56: 4282–4285. Available: http://dx.doi.org/10.1002/anie.201611819. Eprint version Post-print DOI 10.1002/anie.201611819 Publisher Wiley Journal Angewandte Chemie International Edition Rights This is the peer reviewed version of the following article: Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C-O and C-C Bond Activation, which has been published in final form at http:// doi.org/10.1002/anie.201611819. This article may be used for non-commercial purposes in accordance With Wiley Terms and Conditions for self-archiving. Download date 23/09/2021 09:53:43 Link to Item http://hdl.handle.net/10754/623218 COMMUNICATION Catalytic Ester and Amide to Amine Interconversion: Nickel- Catalyzed Decarbonylative Amination of Esters and Amides via C- O and C-C Bond Activation Huifeng Yue[a], Lin Guo[a], Hsuan-Hung Liao[a], Yunfei Cai[a], Chen Zhu[a], and Magnus Rueping[a,b]* Abstract: An efficient nickel catalyzed decarbonylative amination reaction of aryl and heteroaryl esters has been achieved for the first a) Schmidt reaction; Curtius, Hoffmann, Lossen rearrangement time. -

Use of Chemically Modified Poly(Ethylene Terephthalate)-G- (Acryl Amide) Fibers for Α-Amylase Immobilization
http://www.e-polymers.org e-Polymers 2007, no. 070 ISSN 1618-7229 Use of chemically modified poly(ethylene terephthalate)-g- (acryl amide) fibers for α-amylase immobilization Zülfikar Temoçin, Mustafa Yiğitoğlu* Kırıkkale University, Science and Arts Faculty, Chemistry Department, Yahşihan, 71450 Kırıkkale, Turkey; Fax: +90-318-3572461; [email protected]. (Received: 12 April, 2007; published: 30 June, 2007) Abstract: Acryl amide grafted Poly(ethylene terephthalate) (AAm-g-PET) fiber was used for covalent coupling of α-amylase. The amide groups of Poly(acryl amide) were converted to the amine groups by Hofmann degradation reaction. The amine groups were activated by glutaraldehyde, before coupling of the enzyme. The free α-amylase and immobilized α-amylase were characterized by determining the activity profile as function of pH, temperature, thermal stability and storage stability. For the immobilized α-amylase, operational stability was also determined. The immobilization of α-amylase on support caused the optimal reaction pH to shift from 5 to 6. The maximum activity of the free and immobilized enzymes occurred at 0 50 C. Km for the immobilized system was higher than that for the free enzyme. The activity of the free enzyme ended in 30 days, whereas the activity of the immobilized enzyme lasted for 60 days at storage conditions. α-Amylase immobilized on matrix maintained 40% of its original activity after 30 times of repeated use. Introduction α-Amylase is a kind of starch-hydrolyzed enzyme. It is an endo-enzyme that can cut α-1,4 glucosidic linkage of starch molecules randomly to form oligo with different molecular weight such as glucose, cane sugar and straight chain oligo, rapidly reducing the viscosity of liquid and has been used in big volume starch hydrolysis industry [1]. -

1 Chapter 3: Organic Compounds: Alkanes and Cycloalkanes
Chapter 3: Organic Compounds: Alkanes and Cycloalkanes >11 million organic compounds which are classified into families according to structure and reactivity Functional Group (FG): group of atoms which are part of a large molecule that have characteristic chemical behavior. FG’s behave similarly in every molecule they are part of. The chemistry of the organic molecule is defined by the function groups it contains 1 C C Alkanes Carbon - Carbon Multiple Bonds Carbon-heteroatom single bonds basic C N C C C X X= F, Cl, Br, I amines Alkenes Alkyl Halide H C C C O C C O Alkynes alcohols ethers acidic H H H C S C C C C S C C H sulfides C C thiols (disulfides) H H Arenes Carbonyl-oxygen double bonds (carbonyls) Carbon-nitrogen multiple bonds acidic basic O O O N H C H C O C Cl imine (Schiff base) aldehyde carboxylic acid acid chloride O O O O C C N C C C C O O C C nitrile (cyano group) ketones ester anhydrides O C N amide opsin Lys-NH2 + Lys- opsin H O H N rhodopsin H 2 Alkanes and Alkane Isomers Alkanes: organic compounds with only C-C and C-H single (s) bonds. general formula for alkanes: CnH(2n+2) Saturated hydrocarbons Hydrocarbons: contains only carbon and hydrogen Saturated" contains only single bonds Isomers: compounds with the same chemical formula, but different arrangement of atoms Constitutional isomer: have different connectivities (not limited to alkanes) C H O C4H10 C5H12 2 6 O OH butanol diethyl ether straight-chain or normal hydrocarbons branched hydrocarbons n-butane n-pentane Systematic Nomenclature (IUPAC System) Prefix-Parent-Suffix -

Carboxylic Acids a Carbonyl with One OH Attached Is Called a Carboxylic
Carboxylic Acids A carbonyl with one OH attached is called a carboxylic acid One important property of carboxylic acids is the acidity O O pKa = 4.7 B BH H3C OH H3C O acetic acid Upon deprotonation a carboxylate is formed Nomenclature There are two important guidelines to know about carboxylic acids: 1) The carboxylic acid has the highest priority in naming 2) In common names, the point of substitution is labeled by the Greek letter counting from the carbonyl " O OH # ! This naming is common practice amongst organic chemists, e,g, substitution at α-carbon Examples (E)-2-pentenoic acid 3-bromo-2-methylpentanoic acid Or β-bromo-α-methylpentanoic acid (common) Common Names Many acid compounds have a common name Most prevalent amongst these are aromatic compounds Physical Properties Most physical properties of carboxylic acids are a result of hydrogen bonding The carboxylic acids form dimers through hydrogen bonding This hydrogen bonding causes a higher melting point and boiling point compared to compounds of similar molecular weight Acidity As observed previously, carboxylic acids are far more acidic than alcohols This is due to the stability of the anion formed after deprotonation Can also stabilize anion through inductive effects Polar bonds near anion source can stabilize negative charge (inductive effect) Consider the acetate group again: Carboxylate Salts Upon deprotonation of a carboxylic acid obtain a carboxylate salt This salt has different physical properties than the acid form (similar to the difference in amine salts discussed -

Organic Chemistry Test for Amide Group
Chemistry Organic Chemistry Test for Amide Group General Aim Method To test for amide-containing organic compounds Detection of the presence of amides using special through the chemical detection of amide groups. and specic reagents. Learning Objectives (ILOs) Dene and determine organic compounds containing amide groups theoretically through their chemical structure. Classify organic compounds containing amide groups into aliphatic and aromatic. Compare between amide groups and other functional groups in terms of chemical structures, properties and reactions. Identify amides experimentally. Select the appropriate reagents to dierentiate between amides and other organic compounds. Theoretical Background/Context - Amides are organic compounds that are considered as acid amides. Although the main dominant class of amides is the carboxyamide, phosphamides and sulphamides are also abundant. - Carboxyamindes possess at least one amide group (-CONH-).The functional group a carbonyl group (C=O) and amino group (-NH2) linked together. Amides (R – CON – R2’) are characterized by the possibility of having its R groups as a terminal H in their structure. In other words, the N can be linked with one or two H atoms instead of R groups. - The role that amides play in our real life is controlled by their properties. Amides are used in various applications in dierent elds such as food industry, pharmaceutics, etc. First: Preparation of Amides The simplest form of amide is prepared from ammonia, where a hydrogen atom is replaced by an acyl group. The product is described as a primary amide where its general formula is represented as R – CO – NH2. In the same way, secondary and tertiary amides can be prepared from primary and secondary amines, respectively. -
Chapter 6 Amines and Amides
Chapter 6 Amines and Amides Chapter 6 Amines and Amides Chapter Objectives: • Learn to recognize the amine and amide functional groups. • Learn the IUPAC system for naming amines and amides. • Learn the important physical properties of the amines and amides. • Learn the major chemical reactions of amines and amides, and learn how to predict the products of amide synthesis and hydrolysis reactions. • Learn some of the important properties of condensation polymers, especially the polyamides. Mr. Kevin A. Boudreaux Angelo State University CHEM 2353 Fundamentals of Organic Chemistry Organic and Biochemistry for Today (Seager & Slabaugh) www.angelo.edu/faculty/kboudrea Nitrogen-Containing Functional Groups • Nitrogen is in Group V of the periodic table, and in most of its compounds, it has three single bonds and one lone pair: N • In this chapter, we will take a look at two functional groups which contain nitrogen atoms connected to carbons: the amines and the amides. O RR''N RCN R' R' R" Amine Amide 2 Chapter 6 Amines and Amides Classification and Nomenclature of Amines 3 Amines • Amines and amides are abundant in nature. They are a major component of proteins and enzymes, nucleic acids, alkaloid drugs, etc. (Alkaloids are N- containing, weakly basic organic compounds; thousands of these substances are known.) • Amines are organic derivatives of ammonia, NH3, in which one or more of the three H’s is replaced by a carbon group. • Amines are classified as primary (1°), secondary (2°), or tertiary (3°), depending on how many carbon groups are connected to the nitrogen atom. HHN RHN RHN RR''N H H R' R' Ammonia 1° Amine 2° Amine 3° Amine 4 Chapter 6 Amines and Amides Examples: Classifying Amines • Classify the following amines as primary (1°), secondary (2°), or tertiary (3°). -

Nucleophilic Reactions of Carboxylic Acid Derivatives
Nucleophilic Reactions of Carboxylic Acid Derivatives Chapter 22 Anhydrides and Amides Types of anhydrides: Types of amides: Unsubstituted Monosubstituted Disubstituted Lactones, Lactams and Nitriles Resonance of Carboxylic Acid Derivatives • Three resonance structures stabilize carboxylic acid derivatives (RCOZ) by delocalizing electron density. • The more resonance structures 2 and 3 contribute to the resonance hybrid, the more stable RCOZ is: Stability of Carboxylic Acid Derivatives • Because the basicity of Z determines the relative stability of the carboxylic acid derivatives, the following stability order results: • In summary, as the basicity of Z increases, the stability of RCOZ increases because of added resonance stabilization. Resonance of Carboxylic Acid Derivatives • The structure and bonding of nitriles is very different from that of other carboxylic acid derivatives, and resembles the C–C triple bond of alkynes. • The carbon atom on the C≡N group is sp hybridized, making it linear with a bond angle of 180°. • The triple bond consists of one σ and two π bonds. 7 Nomenclature—Acid Chlorides • For acyclic acid chlorides: change the suffix –ic acid of the parent carboxylic acid to the suffix –yl chloride; or • When the –COCl group is bonded to a ring: change the suffix – carboxylic acid to –carbonyl chloride. Nomenclature—Anhydrides • Symmetrical anhydrides are named by changing the acid ending of the carboxylic acid to the word anhydride. • Mixed anhydrides, which are derived from two different carboxylic acids, are named by alphabetizing the names for both acids and replacing the word acid with the word anhydride. 9 Composition of Anhydrides • Anhydride means without water. • Removing one molecule of water from two molecules of carboxylic acid forms an anhydride. -

Hydrides As Reducing Agents
Hydrides as Reducing Agents Lithium aluminum hydride (LiAlH4) is a strong reducing agent. It will donate hydride (“H-”) to any C=O containing functional group. Examples: + 2. H3O 1. LiAlH4 (or just H2O) aldehyde primary alcohol 1. LiAlH4 2. H2O ketone secondary alcohol Hydrides as Reducing Agents Lithium aluminum hydride (LiAlH4) is a strong reducing agent. It will reduce almost any C=O containing functional group to an alcohol. Example: 1. LiAlH4 2. H2O ester One and then another equivalent equivalent adds, of H- adds, unavoidably. Reduced by LiAlH4 to an alcohol: aldehyde ketone carboxylic acid ester acyl halide Double Addition of Hydride to Carboxylic Acids and Derivatives Why? Ketones and aldehydes are more electrophilic than acids, esters and acyl halides. As soon as a ketone or aldehyde is Lone pair donation by generated, it is immediately reduced oxygen reduces partial again. positive charge on C=O carbon. Reduced by LiAlH4 to an alcohol: aldehyde ketone carboxylic acid ester acyl halide Hydrides as Reducing Agents Exception: LiAlH4 reduces amides to amines. Examples: 1. LiAlH4 Mechanism depends slightly on whether amide has an N-H or 2. H2O not. But the result is the same. 1. LiAlH4 2. H2O Reduced by LiAlH4 to an alcohol: aldehyde ketone carboxylic acid ester acyl halide Hydrides as Reducing Agents Sodium borohydride (NaBH4) is a mild reducing agent. It is only capable of reducing aldehydes and ketones. NaBH4 NaBH4 isn’t as basic as LiAlH4, EtOH so reaction can be conducted in protic solvent, and separate workup step isn’t essential. aldehyde primary alcohol NaBH4 EtOH Reduced by NaBH4: ketone secondary alcohol aldehyde ketone Biological Cofactors as Redox Agents LiAlH4 isn’t used in biology, but biological reductants are mechanistically similar.