Naming Amines and Amides (Rules)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani
Reduction of Organic Functional Groups Using Hypophosphites Rim Mouselmani To cite this version: Rim Mouselmani. Reduction of Organic Functional Groups Using Hypophosphites. Other. Univer- sité de Lyon; École Doctorale des Sciences et de Technologie (Beyrouth), 2018. English. NNT : 2018LYSE1241. tel-02147583v2 HAL Id: tel-02147583 https://tel.archives-ouvertes.fr/tel-02147583v2 Submitted on 5 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THESE de DOCTORAT DE L’UNIVERSITE DE LYON EN COTUTELLE AVEC L'UNIVERSITÉ LIBANAISE opérée au sein de l’Université Claude Bernard Lyon 1 École Doctorale de Chimie-École Doctorale des Sciences et Technologies Discipline : Chimie Soutenue publiquement le 07/11/2018, par Rim MOUSELMANI Reduction of Organic Functional Groups Using Hypophosphites Devant le jury composé de Mme. Micheline DRAYE Université Savoie Mont Blanc Rapporteure M. Mohammad ELDAKDOUKI Université Arabe de Beyrouth Rapporteur Mme. Emmanuelle SCHULZ Université Paris 11 examinatrice M. Abderrahmane AMGOUNE Université Lyon 1 Président M. Mahmoud FARAJ Université Internationale Libanaise examinateur Mme. Estelle MÉTAY Université Lyon 1 Directrice de thèse M. Ali HACHEM Université Libanaise Directeur de thèse M. Marc LEMAIRE Université Lyon 1 Membre invité M. -

Amide, and Paratoluenesulfonamide on the Amide of Silver,On the Imides
68 CHEMISTRY: E. C. FRANKLIN METALLIC SALTS OF AMMONO ACIDS By Edward C. Franklin DEPARTMENT OF CHEMISTRY, STANFORD UNIVERSITY Presented to the Academy, January 9. 1915 The Action of Liquid-Ammonia Solutions of Ammono Acids on Metallic Amides, Imides, and Nitrides. The acid amides and imides, and the metallic derivatives of the acid amides and imides are the acds, bases, and salts respectively of an ammonia system of acids, bases, and salts.1 Guided by the relationships implied in the above statement Franklin and Stafford were able to prepare potassium derivatives of a considerable number of acid amides by the action of potassium amide on certain acid amides in solution in liquid ammonia. That is to say, an ammono base, potassium amide, was found to react with ammono acids in liquid ammonia to form ammono salts just as the aquo base, potassium hydrox- ide, acts upon aquo acids in water solution to form aquo salts. Choos- ing, for example, benzamide and benzoic acid as representative acids of the two systems, the analogous reactions taking place respectively in liquid ammonia and water are represented by the equations: CH6CONH2+KNH2 = C6H5CONHK + NHs. CseHCONH2 + 2KNH2 = CIHsCONK2 + 2NH3. CH6tCOOH + KOH = CIH6COOK + H2O. The ammono acid, since it is dibasic, reacts with either one or two molecules of potassium amide to form an acid and a neutral salt. Having thus demonstrated the possibility of preparing ammono salts of potassium by the interaction of potassium amide and acid amides in liquid ammonia solution, it was further found that ammono salts of the heavy metals may be prepared by the action of liquid ammonia solutions of ammono acids on insoluble metallic amides, imides, and nitrides-that is, by reactions which are analogous to the formation of aquo salts in water by the action of potassium hydroxide on insoluble metallic hydroxides and oxides. -

Amide Activation: an Emerging Tool for Chemoselective Synthesis
Featuring work from the research group of Professor As featured in: Nuno Maulide, University of Vienna, Vienna, Austria Amide activation: an emerging tool for chemoselective synthesis Let them stand out of the crowd – Amide activation enables the chemoselective modification of a large variety of molecules while leaving many other functional groups untouched, making it attractive for the synthesis of sophisticated targets. This issue features a review on this emerging field and its application in total synthesis. See Nuno Maulide et al., Chem. Soc. Rev., 2018, 47, 7899. rsc.li/chem-soc-rev Registered charity number: 207890 Chem Soc Rev View Article Online REVIEW ARTICLE View Journal | View Issue Amide activation: an emerging tool for chemoselective synthesis Cite this: Chem. Soc. Rev., 2018, 47,7899 Daniel Kaiser, Adriano Bauer, Miran Lemmerer and Nuno Maulide * It is textbook knowledge that carboxamides benefit from increased stabilisation of the electrophilic carbonyl carbon when compared to other carbonyl and carboxyl derivatives. This results in a considerably reduced reactivity towards nucleophiles. Accordingly, a perception has been developed of amides as significantly less useful functional handles than their ester and acid chloride counterparts. Received 27th April 2018 However, a significant body of research on the selective activation of amides to achieve powerful DOI: 10.1039/c8cs00335a transformations under mild conditions has emerged over the past decades. This review article aims at placing electrophilic amide activation in both a historical context and in that of natural product rsc.li/chem-soc-rev synthesis, highlighting the synthetic applications and the potential of this approach. Creative Commons Attribution 3.0 Unported Licence. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Chapter 18 Amines in Order for a Drug to Be Effective Orally, It Generally
Chapter 18 Amines In order for a drug to be effective orally, it generally has to be reasonable soluble in water so that it can be transported through the blood. Since amines are weak bases, they are often converted to salts with some acid and therefore may oral drugs have amine salts as part of their structure. One reason for their presence is that they confer some water solubility to the drug. The three-dimensional models show the shapes of amine molecules; notice the lone pair of electrons on nitrogen is not shown but affects the geometry about the nitrogen Primary, secondary and tertiary amines have 1, 2 or 3 alkyl groups attached to nitrogen. In these cases the alkyl group is the methyl In the IUPAC system, STEP 1 Name the longest carbon chain bonded to the N atoms as alkanamines by replacing e of the alkane name with amine. STEP 2 Number the carbon chain to locate the amine group and any substituents. N,N-Dimethylethanamine aminoethane 2-aminopropane 2-(N,N-dimethylamino)ethane 1-(N-methylamino)propane 2-(N-methylamino)butane Amines can also be names as groups attached to a hydrocarbon Aminobenzene is called aniline NH2 C H H C C C C H H C H NH2 NH2 NH CH3 Cl aniline 3-chloroaniline N-methylaniline aminobenzene 3-chloroaminobenzene N-methylaminobenzene Properties of amines The boiling points of amines are higher than alkanes of similar mass lower than alcohols of similar mass Amines are soluble in water if they have 1 to 5 carbon atoms; the N atom forms hydrogen bonds with the polar O—H bond in water An amine salt forms when an amine -
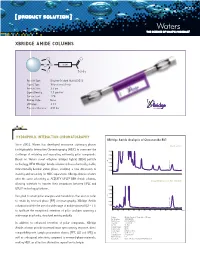
Xbridge Amide Columns
[ PRODUCT SOLUTION ] XBRIDGE AMIDE COLUMNS O O O Si Linker O NH 2 Particle Type: Ethylene Bridged Hybrid [BEH] Ligand Type: Trifunctional Amide Particle Size: 3.5 µm Ligand Density: 7.5 µmol/m2 Carbon Load: 12% Endcap Style: None pH Range: 2-11 Pressure Tolerance: 400 bar HYDROPHILIC INTERacTION CHROMATOGRAPHY XBridge Amide Analysis of Ginsenoside Rb1 Since 2003, Waters has developed innovative stationary phases Orento extract for Hydrophilic Interaction Chromatography [HILIC] to overcome the 0.010 challenge of retaining and separating extremely polar compounds. 0.008 Based on Waters novel ethylene bridged hybrid [BEH] particle 0.006 AU technology, NEW XBridge™ Amide columns utilize a chemically stable, 0.004 0.002 Ginsenoside Rb1 trifunctionally-bonded amide phase, enabling a new dimension in 0.000 stability and versatility for HILIC separations. XBridge Amide columns offer the same selectivity as ACQUITY UPLC® BEH Amide columns, 20 µg/mL Ginsenoside Rb1 standard allowing scientists to transfer their separations between HPLC and 0.010 ® UPLC technology platforms. 0.008 0.006 Designed to retain polar analytes and metabolites that are too polar AU 0.004 to retain by reversed-phase [RP] chromatography, XBridge Amide 0.002 columns facilitate the use of a wide range of mobile phase pH [2 – 11] 0.000 Ginsenoside Rb1 to facilitate the exceptional retention of polar analytes spanning a 0.00. 5 1.01. 5 2.02. 5 3.03. 5 4.04. 5 5.05. 5 6.06. 5 min wide range in polarity, structural moiety and pKa. Column: XBridge Amide, 3.5 µm, 4.6 x 150 mm Part Number: 186004869 Mobile Phase : 80/20 ACN/H2O In addition to enhanced retention of polar compounds, XBridge Flow Rate: 1.4 mL/min Inj. -

In This Handout, All of Our Functional Groups Are Presented As Condensed Line Formulas, 2D and 3D Formulas and with Nomenclature Prefixes and Suffixes (If Present)
In this handout, all of our functional groups are presented as condensed line formulas, 2D and 3D formulas and with nomenclature prefixes and suffixes (if present). Organic names are built on a foundation of alkanes, alkenes and alkynes. Those examples are presented first and you need to know those rules. The strategies can be found in Chapter 4 of our textbook (alkanes: pages 93-98, cycloalkanes 102-104, alkenes: pages 104-110, alkynes: pages 112-113 and combinations of all of them 113-115). After introducing examples of alkanes, alkenes, alkynes and combinations of them, the functional groups are presented in order of priority. A few nomenclature examples are provided for each of the functional groups. Examples of the various functional groups are presented on pages 115-135 in the textbook. Two overview pages are on pages 136-137. Some functional groups have a suffix name when they are the highest priority functional group and a prefix name when they are not the highest priority group, and these are added to the skeletal names with identifying numbers and stereochemistry terms (E and Z for alkenes, R and S for chiral centers and cis and trans for rings). Several low priority functional groups only have a prefix name. A few additional special patterns are shown on pages 98-102. The only way to learn this topic is practice (over and over). The best practice approach is to actually write out the names (on an extra piece of paper or on a white board, and then do it again). The same functional groups are used throughout the entire course. -

Efficient Synthesis of Novel Pyridine-Based Derivatives Via
molecules Article Efficient Synthesis of Novel Pyridine-Based Derivatives via Suzuki Cross-Coupling Reaction of Commercially Available 5-Bromo-2-methylpyridin-3-amine: Quantum Mechanical Investigations and Biological Activities Gulraiz Ahmad 1, Nasir Rasool 1,*, Hafiz Mansoor Ikram 1, Samreen Gul Khan 1, Tariq Mahmood 2, Khurshid Ayub 2, Muhammad Zubair 1, Eman Al-Zahrani 3, Usman Ali Rana 4, Muhammad Nadeem Akhtar 5 and Noorjahan Banu Alitheen 6,* 1 Department of Chemistry, Government College University Faisalabad, Faisalabad 38000, Pakistan; [email protected] (G.A.); [email protected] (H.M.I.); [email protected] (S.G.K.); [email protected] (M.Z.) 2 Department of Chemistry, COMSATS Institute of Information Technology, University Road, Tobe Camp, 22060 Abbottabad, Pakistan; [email protected] (T.M.); [email protected] (K.A.) 3 Chemistry Department, Faculty of Science, Taif University, 888-Taif, Saudi Arabia; [email protected] 4 Sustainable Energy Technologies Center, College of Engineering, P.O. Box 800, King Saud University, Riyadh 11421, Saudi Arabia; [email protected] 5 Faculty of Industrial Sciences & Technology, University Malaysia Pahang, Lebuhraya Tun, Razak 26300, Kuantan Pahang, Malaysia; [email protected] 6 Faculty of Biotechnology and Biomolecular Sciences, University Putra Malaysia, Serdang, Selangor Darul Ehsan 43400, Malaysia * Correspondence: [email protected] (N.R.); [email protected] (N.B.A.); Tel.: +92-332-7491790 (N.R.); +60-3-8946-7471 (N.B.A.); Fax: +92-41-9201032 (N.R.); +60-3-8946-7510 (N.B.A.) Academic Editor: Diego Muñoz-Torrero Received: 20 November 2016; Accepted: 6 January 2017; Published: 27 January 2017 Abstract: The present study describes palladium-catalyzed one pot Suzuki cross-coupling reaction to synthesize a series of novel pyridine derivatives 2a–2i, 4a–4i. -

United States Patent Office Patented Dec
3,114,704 United States Patent Office Patented Dec. 17, 1963 2 This invention is based in part on the discovery that 3,114,704 by hydrogenating the nitrile pitch and combining the ORE FLOTATION COLLECTOR AND ORE hydrogenated pitch in certain proportions with primary FOTAEON PROCESS fatty monomaines that an ore flotation collector can be Robert S. Kaufman, Paios Heights, Robert E. Baarson, 5 produced which is of equal or greater effectiveness to the Chicago, and Harold B. Treweek, Park Forest, ii., assignors to Armour and Corinpasay, Chicago, I., a straight primary amines. Since the hydrogenated nitrile corporation of Delaware pitch may comprise as much as 40 to 60% of the col No Drawing. Filed Apr. 20, 1961, Ser. No. 104,242 lector, the cost of the collector is greatly reduced. Fur 6 Claims. (C. 299-166) thermore, when the preferred proportions are employed, O the collector will not only be of substantially lower cost, This invention relates to an ore flotation collector but it will also have improved selectivity and/or collector which has particular utility in the froth flotation of silica strength. containing phosphate ores, and therefore the invention The nitrile pitch as produced, for example, by the pro also relates to a phosphate ore flotation process. cedures described in U.S. Patent Nos. 2,314,894 and At the present time primary fatty monoamines are 5 2,808,426, will contain nitriles as the principal component widely used in the froth flotation of silica containing and Will be composed of compounds and polymers there phosphate ores. -

Catalytic Carbonylation of Amines And
CATALYTIC CARBONYLATION OF AMINES AND DIAMINES AS AN ALTERNATIVE TO PHOSGENE DERIVATIVES: APPLICATION TO SYNTHESES OF THE CORE STRUCTURE OF DMP 323 AND DMP 450 AND OTHER FUNCTIONALIZED UREAS By KEISHA-GAY HYLTON A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2004 Copyright 2004 by Keisha-Gay Hylton Dedicated to my father Alvest Hylton; he never lived to celebrate any of my achievements but he is never forgotten. ACKNOWLEDGMENTS A number of special individuals have contributed to my success. I thank my mother, for her never-ending support of my dreams; and my grandmother, for instilling integrity, and for her encouragement. Special thanks go to my husband Nemanja. He is my confidant, my best friend, and the love of my life. I thank him for providing a listening ear when I needed to “discuss” my reactions; and for his support throughout these 5 years. To my advisor (Dr. Lisa McElwee-White), I express my gratitude for all she has taught me over the last 4 years. She has shaped me into the chemist I am today, and has provided a positive role model for me. I am eternally grateful. I, of course, could never forget to mention my group members. I give special mention to Corey Anthony, for all the free coffee and toaster strudels; and for helping to keep the homesickness at bay. I thank Daniel for all the good gossip and lessons about France. I thank Yue Zhang for carbonylation discussions, and lessons about China. -

Detection of Atmospheric Gaseous Amines and Amides by a High-Resolution Time-Of-flight Chemical Ionization Mass Spectrometer with Protonated Ethanol Reagent Ions
Atmos. Chem. Phys., 16, 14527–14543, 2016 www.atmos-chem-phys.net/16/14527/2016/ doi:10.5194/acp-16-14527-2016 © Author(s) 2016. CC Attribution 3.0 License. Detection of atmospheric gaseous amines and amides by a high-resolution time-of-flight chemical ionization mass spectrometer with protonated ethanol reagent ions Lei Yao1, Ming-Yi Wang1,a, Xin-Ke Wang1, Yi-Jun Liu1,b, Hang-Fei Chen1, Jun Zheng2, Wei Nie3,4, Ai-Jun Ding3,4, Fu-Hai Geng5, Dong-Fang Wang6, Jian-Min Chen1, Douglas R. Worsnop7, and Lin Wang1,4 1Shanghai Key Laboratory of Atmospheric Particle Pollution and Prevention (LAP3), Department of Environmental Science & Engineering, Fudan University, Shanghai 200433, China 2Jiangsu Key Laboratory of Atmospheric Environment Monitoring and Pollution Control, Nanjing University of Information Science & Technology, Nanjing 210044, China 3Joint International Research Laboratory of Atmospheric and Earth System Sciences, School of Atmospheric Science, Nanjing University, Nanjing 210023, China 4Collaborative Innovation Center of Climate Change, Nanjing 210023, China 5Shanghai Meteorology Bureau, Shanghai 200135, China 6Shanghai Environmental Monitoring Center, Shanghai 200030, China 7Aerodyne Research, Billerica, MA 01821, USA anow at: Center for Atmospheric Particle Studies, Carnegie Mellon University, Pittsburgh, PA 15213, USA bnow at: Pratt School of Engineering, Duke University, Durham, NC 27705, USA Correspondence to: Lin Wang([email protected]) Received: 7 June 2016 – Published in Atmos. Chem. Phys. Discuss.: 22 June 2016 Revised: 15 October 2016 – Accepted: 3 November 2016 – Published: 23 November 2016 Abstract. Amines and amides are important atmospheric concentrations of amines ranged from a few parts per trillion organic-nitrogen compounds but high time resolution, highly by volume to hundreds of parts per trillion by volume, con- sensitive, and simultaneous ambient measurements of these centrations of amides varied from tens of parts per trillion by species are rather sparse. -

COMMUNICATION Catalytic Ester and Amide To
Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C−O and C−C Bond Activation Item Type Article Authors Yue, Huifeng; Guo, Lin; Liao, Hsuan-Hung; Cai, Yunfei; Zhu, Chen; Rueping, Magnus Citation Yue H, Guo L, Liao H-H, Cai Y, Zhu C, et al. (2017) Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C−O and C −C Bond Activation. Angewandte Chemie International Edition 56: 4282–4285. Available: http://dx.doi.org/10.1002/anie.201611819. Eprint version Post-print DOI 10.1002/anie.201611819 Publisher Wiley Journal Angewandte Chemie International Edition Rights This is the peer reviewed version of the following article: Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C-O and C-C Bond Activation, which has been published in final form at http:// doi.org/10.1002/anie.201611819. This article may be used for non-commercial purposes in accordance With Wiley Terms and Conditions for self-archiving. Download date 23/09/2021 09:53:43 Link to Item http://hdl.handle.net/10754/623218 COMMUNICATION Catalytic Ester and Amide to Amine Interconversion: Nickel- Catalyzed Decarbonylative Amination of Esters and Amides via C- O and C-C Bond Activation Huifeng Yue[a], Lin Guo[a], Hsuan-Hung Liao[a], Yunfei Cai[a], Chen Zhu[a], and Magnus Rueping[a,b]* Abstract: An efficient nickel catalyzed decarbonylative amination reaction of aryl and heteroaryl esters has been achieved for the first a) Schmidt reaction; Curtius, Hoffmann, Lossen rearrangement time.