Invited Review Compartmentation of the Granular Layer of the Cerebellum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets
THE JOURNAL OF COMPARATIVE NEUROLOGY 369~345-360 ( 1996) Ultrastructural Study of the Granule Cell Domain of the Cochlear Nucleus in Rats: Mossy Fiber Endings and Their Targets DIANA L. WEEDMAN, TAN PONGSTAPORN, AND DAVID K. RYUGO Center for Hearing Sciences, Departments of Otolaryngoloby-Head and Neck Surgery and Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland 2 1205 ABSTRACT The principal projection neurons of the cochlear nucleus receive the bulk of their input from the auditory nerve. These projection neurons reside in the core of the nucleus and are surrounded by an external shell, which is called the granule cell domain. Interneurons of the cochlear granule cell domain are the target for nonprimary auditory inputs, including projections from the superior olivary complex, inferior colliculus, and auditory cortex. The granule cell domain also receives projections from the cuneate and trigeminal nuclei, which are first-order nuclei of the somatosensory system. The cellular targets of the nonprimary projections are mostly unknown due to a lack of information regarding postsynaptic profiles in the granule cell areas. In the present paper, we examined the synaptic relationships between a heterogeneous class of large synaptic terminals called mossy fibers and their targets within subdivisions of the granule cell domain known as the lamina and superficial layer. By using light and electron microscopic methods in these subdivisions, we provide evidence for three different neuron classes that receive input from the mossy fibers: granule cells, unipolar brush cells, and a previously undescribed class called chestnut cells. The distinct synaptic relations between mossy fibers and members of each neuron class further imply fundamentally separate roles for processing acoustic signals. -

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

FIRST PROOF Cerebellum
Article Number : EONS : 0736 GROSS ANATOMY Cerebellum Cortex The cerebellar cortex is an extensive three-layered sheet with a surface approximately 15 cm laterally THE HUMAN CEREBELLUM (‘‘little brain’’) is a and 180 cm rostrocaudally but densely folded around significant part of the central nervous system both three pairs of nuclei. The cortex is divided into three in size and in neural structure. It occupies approxi- transverse lobes: Anterior and posterior lobes are mately one-tenth of the cranial cavity, sitting astride separated by the primary fissure, and the smaller the brainstem, beneath the occipital cortex, and flocculonodular lobe is separated by the poster- contains more neurons than the whole of the cerebral olateral fissure (Fig. 1). The anterior and posterior cortex. It consists of an extensive cortical sheet, lobes are folded into a number of lobules and further densely folded around three pairs of nuclei. The folded into a series of folia. This transverse organiza- cortex contains only five main neural cell types and is tion is then divided at right angles into broad one of the most regular and uniform structures in the longitudinal regions. The central vermis, named for central nervous system (CNS), with an orthogonal its worm-like appearance, is most obvious in the ‘‘crystalline’’ organization. Major connections are posterior lobe. On either side is the paravermal or made to and from the spinal cord, brainstem, and intermediate cortex, which merges into the lateral sensorimotor areas of the cerebral cortex. hemispheres. The most common causes of damage to the cerebellum are stroke, tumors, or multiple sclerosis. -
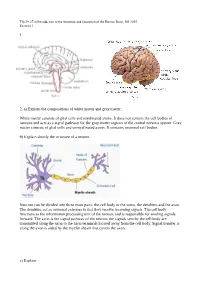
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Control of Cerebellar Granule Cell Output by Sensory-Evoked Golgi Cell Inhibition
Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition Ian Duguid1,2,3, Tiago Branco1,4, Paul Chadderton5, Charlotte Arlt, Kate Powell6, and Michael Häusser3 Wolfson Institute for Biomedical Research and Department of Neuroscience, Physiology, and Pharmacology, University College London, London WC1E 6BT, United Kingdom Edited by Masao Ito, RIKEN Brain Science Institute, Wako, Japan, and approved September 1, 2015 (received for review May 25, 2015) Classical feed-forward inhibition involves an excitation–inhibition Results sequence that enhances the temporal precision of neuronal re- Sensory-Evoked Phasic and Spillover Golgi Cell Inhibition Precedes sponses by narrowing the window for synaptic integration. In Mossy Fiber Excitation in Granule Cells. Cerebellar granule cells the input layer of the cerebellum, feed-forward inhibition is thought receive direct phasic and indirect or “spillover” GABAergic in- to preserve the temporal fidelity of granule cell spikes during mossy put from Golgi cells (6, 16, 20, 21). To investigate the temporal fiber stimulation. Although this classical feed-forward inhibitory cir- dynamics of sensory-evoked inhibition in vivo, we recorded cuit has been demonstrated in vitro, the extent to which inhibition spontaneous and sensory-evoked excitatory (Vhold = −70 mV) shapes granule cell sensory responses in vivo remains unresolved. and inhibitory (Vhold = 0 mV) currents from the same granule Here we combined whole-cell patch-clamp recordings in vivo and cells in Crus II (Fig. 1 A–D). Granule cells were identified based dynamic clamp recordings in vitro to directly assess the impact of on their characteristic electrophysiological properties (Table S1), Golgi cell inhibition on sensory information transmission in the depth from the pial surface (>250 μm), and morphology (Fig. -

Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons
The Journal of Neuroscience, September 1989, g(9): 30303039 Intersegmental Interneurons Can Control the Gain of Reflexes in Adjacent Segments of the Locust by Their Action on Nonspiking Local Interneurons Gilles Laurent and Malcolm Burrows Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, England The gain of local reflexes of one leg of a locust can be altered partmentalized, with synaptic inputs and their associated by mechanosensory inputs generated by movements of or conductance changes restricted to particular branches. In tactile inputs to an adjacent leg. Touching the mesothoracic this way, an individual nonspiking neuron could contribute tarsus, for example, increases the number of spikes that are simultaneously to several local circuits. The inputs from dif- produced by the metathoracic slow extensor tibiae motor ferent intersegmental interneurons could then modulate these neuron and enhances the depolarization of flexor tibiae mo- pathways independently. tor neuron in response to imposed movements of the chor- dotonal organ in the ipsilateral hind femur. The sensory in- Nonspiking interneurons in the metathoracic ganglion of the formation from the middle leg is conveyed directly to locust receive direct inputs from mechanosensory afferents on nonspiking interneurons and motor neurons controlling the one hindleg and are essential elements in local reflex movements movements of the hindleg by a population of mesothoracic of that leg (Laurent and Burrows, 1988; Burrows et al., 1988). intersegmental interneurons (Laurent and Burrows, 1989). They also receive direct inputs from intersegmental intemeu- The metathoracic nonspiking interneurons receive direct in- rons that process the mechanosensory inputs from an ipsilateral puts from receptors on a hindleg and are, therefore, a point middle leg (Laurent and Burrows, 1989). -

Cerebellar Cortical Neuron Responses Evoked from the Spinal Border Cell Tract
Cerebellar cortical neuron responses evoked from the spinal border cell tract. Geborek, Pontus; Spanne, Anton; Bengtsson, Fredrik; Jörntell, Henrik Published in: Frontiers in Neural Circuits DOI: 10.3389/fncir.2013.00157 2013 Link to publication Citation for published version (APA): Geborek, P., Spanne, A., Bengtsson, F., & Jörntell, H. (2013). Cerebellar cortical neuron responses evoked from the spinal border cell tract. Frontiers in Neural Circuits, 7, [157]. https://doi.org/10.3389/fncir.2013.00157 Total number of authors: 4 General rights Unless other specific re-use rights are stated the following general rights apply: Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal Read more about Creative commons licenses: https://creativecommons.org/licenses/ Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. LUND UNIVERSITY PO Box 117 221 00 Lund +46 46-222 00 00 Download date: 04. Oct. 2021 ORIGINAL RESEARCH ARTICLE published: 08 October 2013 NEURAL CIRCUITS doi: 10.3389/fncir.2013.00157 Cerebellar cortical neuron responses evoked from the spinal border cell tract Pontus Geborek, Anton Spanne, Fredrik Bengtsson and Henrik Jörntell* Neural Basis of Sensorimotor Control, Department of Experimental Medical Science, Lund University, Lund, Sweden Edited by: Spinocerebellar systems are likely to be crucial for cerebellar hallmark functions such Egidio D’Angelo, University of Pavia, as coordination. -
Cerebellar Granule Cells Encode the Expectation of Reward Mark J
LETTER doi:10.1038/nature21726 Cerebellar granule cells encode the expectation of reward Mark J. Wagner1*, Tony Hyun Kim1,2*, Joan Savall1, Mark J. Schnitzer1,3 & Liqun Luo1 The human brain contains approximately 60 billion cerebellar identical stimuli and licking responses. In both tasks, reward signals granule cells1, which outnumber all other brain neurons combined. were widespread throughout multiple cerebellar lobules. Tracking Classical theories posit that a large, diverse population of granule the same granule cells over several days of learning revealed that cells allows for highly detailed representations of sensorimotor cells with reward-anticipating responses emerged from those that context, enabling downstream Purkinje cells to sense fine contextual responded at the start of learning to reward delivery, whereas changes2–6. Although evidence suggests a role for the cerebellum in reward-omission responses grew stronger as learning progressed. cognition7–10, granule cells are known to encode only sensory11–13 The discovery of predictive, non-sensorimotor encoding in granule and motor14 context. Here, using two-photon calcium imaging cells is a major departure from the current understanding of these in behaving mice, we show that granule cells convey information neurons and markedly enriches the contextual information available about the expectation of reward. Mice initiated voluntary forelimb to postsynaptic Purkinje cells, with important implications for movements for delayed sugar-water reward. Some granule cells cognitive processing in the cerebellum. responded preferentially to reward or reward omission, whereas Mice voluntarily grasped the handle of a manipulandum (Methods) others selectively encoded reward anticipation. Reward responses and pushed it forward up to 8 mm for delayed receipt of a sucrose-water were not restricted to forelimb movement, as a Pavlovian task evoked reward (Fig. -

The Modifiable Neuronal Network of the Cerebellum
Japanese Journal of Physiology, 34, 781-792, 1984 MINIREVIEW The Modifiable Neuronal Network of the Cerebellum M asao ITO Department of Physiology, Faculty of Medicine, University of Tokyo, Bunkyo-ku, Tokyo, 113 Japan An outstanding feature of the cerebellum known since the classic histological work of Ramon y Cajal is the relative simplicity and highly ordered geometry of its neuronal network. The cerebellar neuronal network consists of five major types of cortical neurons (Purkinje, basket, stellate, Golgi, and granule cells), two major types of afferents (mossy and climbing fibers), and the subcortical cells in the vestibular and cerebellar nuclei. When the neuronal network structure of the cerebellum was comprehensively dissected and described in great detail in the 1960s, and summarized by ECCLES,ITO, and SZ.ENTAGOTHAI[8], it was taken as a challenge to understand more completely the complex functions of the central nervous system using these fundamental studies of the brain as groundwork for future elucidation. As the gap between our understanding of the brain and our knowledge of neuronal networks is in general so large, hope has arisen that cere- bellar physiology may successfully fill the gap rather soon. Rigorous efforts using numerous newly-introduced techniques have been devoted during the past two decades, yielding remarkable progress that meets expectations at least to some extent. A number of problems remain to be solved, however, before we fully understand the cerebellum. It seems important at this point to review the major achievements of the past two decades in order to gain a clearer perspective of cerebellar physiology for the coming decade.