Coumarins and Metabolic Syndrome: Brief Report RESEARCH ARTICLE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

Scopoletin 8-Hydroxylase: a Novel Enzyme Involved in Coumarin
bioRxiv preprint doi: https://doi.org/10.1101/197806; this version posted October 4, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Scopoletin 8-hydroxylase: a novel enzyme involved in coumarin biosynthesis and iron- 2 deficiency responses in Arabidopsis 3 4 Running title: At3g12900 encodes a scopoletin 8-hydroxylase 5 6 Joanna Siwinska1, Kinga Wcisla1, Alexandre Olry2,3, Jeremy Grosjean2,3, Alain Hehn2,3, 7 Frederic Bourgaud2,3, Andrew A. Meharg4, Manus Carey4, Ewa Lojkowska1, Anna 8 Ihnatowicz1,* 9 10 1Intercollegiate Faculty of Biotechnology of University of Gdansk and Medical University of 11 Gdansk, Abrahama 58, 80-307 Gdansk, Poland 12 2Université de Lorraine, UMR 1121 Laboratoire Agronomie et Environnement Nancy- 13 Colmar, 2 avenue de la forêt de Haye 54518 Vandœuvre-lès-Nancy, France; 3INRA, UMR 14 1121 Laboratoire Agronomie et Environnement Nancy-Colmar, 2 avenue de la forêt de Haye 15 54518 Vandœuvre-lès-Nancy, France; 16 4Institute for Global Food Security, Queen’s University Belfast, David Keir Building, Malone 17 Road, Belfast, UK; 18 19 [email protected] 20 [email protected] 21 [email protected] 22 [email protected] 23 [email protected] 24 [email protected] 25 [email protected] 26 [email protected] 27 [email protected] 28 *Correspondence: [email protected], +48 58 523 63 30 29 30 The date of submission: 02.10.2017 31 The number of figures: 9 (Fig. -

Biosynthesis of Daphnetin in Daphne Mezereum L.*
Biosynthesis of Daphnetin in Daphne mezereum L.* Stewart A. Brown Department of Chemistry, Trent University, Peterborough, Ont., Canada K9J 7B8 Z. Naturforsch. 41c, 247—252 (1986); received November 4, 1985 Biosynthesis, Coumarins, Daphne mezereum, Daphnetin Shoots of Daphne mezereum synthesized daphnetin (7,8-dihydroxycoumarin) more efficiently from [2-l4C]umbelliferone (7-hydroxycoumarin) than from [2-l4C]p-coumaric acid, and [2-14C]caf- feic acid was more poorly utilized still. These findings do not support the idea of derivation of daphnetin via hydroxylation of the caffeic acid ring at the 2 position, followed by lactone ring formation; instead they are consistent with the concept of daphnetin formation by an additional hydroxylation of umbelliferone at C-8. Umbelliferone was recovered with little l4C dilution from emulsin-hydrolysed extracts of shoots fed labelled umbelliferone, and TLC of extracts from un treated shoots revealed two substances yielding umbelliferone on hydrolysis. Evidence is pre sented from TLC and HPLC analysis that one of these is skimmin (7-O-ß-D-glucosylumbel- liferone), not previously reported from Daphne. The tracer experiments further support the theory that umbelliferone is the general precursor of coumarins bearing two or more hydroxyl functions on the aromatic ring. Introduction (Thymelaeaceae), a hardy perennial shrub, where it Approximately 70 coumarins with the 7,8 oxygen occurs together with its 7-ß-D-glucoside, daphnin ation pattern' have been reported to be naturally (6b) and daphnetin glucoside (6c), the 8-O-ß-D- occurring [1], over half being furanocoumarins de g lu c o s y la te d is o m e r [1]. -

A Detailed Review on Morphotaxonomy And
Acta Scientific Microbiology (ISSN: 2581-3226) Review Article Volume 1 Issue 6 June 2018 Skimmia anquetilia N.P. Taylor and Airy Shaw A Detailed Review on Morphotaxonomy and Chemoprofiling of Saduf Nissar1, Neelofar Majid1, Aabid M Rather1*, Irshad A Nawchoo1 and GG Mohi-Ud-Din2 1Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, Department of Botany, University of Kashmir, Srinagar, India 2Department of Botany, Government Degree College for Women, Sopore, Baramullah, India *Corresponding Author: Aabid M Rather, Plant Reproductive Biology, Genetic Diversity and Phytochemistry Research Laboratory, DepartmentReceived: April of Botany, 20, 2018; University Published: of Kashmir, May 28, Srinagar, 2018 India. Abstract - In recent times, medicinal plants have attracted huge attention due to their diverse range of biological and therapeutic proper Skimmia ties. Evidences have been accumulated since ages to demonstrate promising potential of medicinal plants used in various traditional, anquetilia complementary, and alternative systems with the ever-increasing interest of today’s population towards natural products, Rutaceae N.P. Taylor and Airy Shaw emerged out to be one of the most eye-catching plant bearing multiple medicinal properties. It is a perennial aromatic evergreen shrub belonging to family . Pharmacological studies have demonstrated significant action - of different extracts as antimicrobial, anti-inflammatory agents, among others, supporting some of its popular uses. An attempt has S. anquetilia been made in this review article to provide an up-to-date overview of the morphological parameters, taxonomic features, distribu tion pattern, traditional uses, as well as the phytochemistry and biological activities of . The present review provides insights for future research aiming for both ethnopharmacological validation of its popular use and its exploration as a new source ofKeywords herbal drugs: Skimmia and/or anquetilia; bioactive Rutaceaenatural products. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -
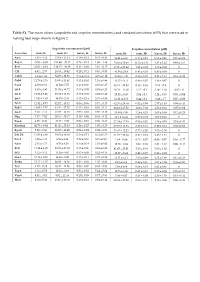
(Scopoletin and Scopolin Concentrations) and Standard Deviations (±SD) That Were Used in Making Heat Maps Shown in Figure 2
Table S1. The mean values (scopoletin and scopolin concentrations) and standard deviations (±SD) that were used in making heat maps shown in Figure 2. Scopoletin concentration [µM] Scopolin concentration [µM] Accession roots, H- roots, H+ leaves, H- leaves, H+ roots, H- roots, H+ leaves, H- leaves, H+ An-1 4.91 ± 4.12 15.19 ± 11.34 0.10 ± 0.12 0.45 ± 0.24 14.81 ± 4.05 0.13 ± 0.12 0.68 ± 0.41 0.05 ± 0.08 Bay-0 41.93 ± 6.58 151.90 ± 30.12 0.29 ± 0.18 1.99 ± 1.46 38.95 ± 10.69 07.15 ± 6.36 3.81 ± 1.22 0.88 ± 1.53 Br-0 35.01 ± 9.48 139.27 ± 49.38 0.31 ± 0.09 2.61 ± 0.27 67.26 ± 23.82 1.22 ± 0.08 1.65 ± 0.08 0 C24 4.92 ± 2.67 16.63 ± 10.42 0.20 ± 0.11 0.83 ± 0.55 41.36 ± 24.8 0.43 ± 0.19 0.85 ± 0.59 0 Can-0 3.14 ± 1.89 64.03 ± 84.91 0.14 ± 0.14 0.37 ± 0.12 34.63 ± 4.85 0.56 ± 0.05 2.23 ± 1.24 0.19 ± 0.33 Col-0 11.79 ± 3.16 29.60 ± 11.41 0.31 ± 0.41 1.16 ± 0.99 11.47 ± 3.41 0.46 ± 0.07 1.48 ± 0.67 0 Cvi-1 4.58 ± 0.62 11.54 ± 7.57 0.19 ± 0.10 0.61 ± 0.27 35.41 ± 32.83 0.35 ± 0.16 2.15 ± 0.8 0 Eil-0 8.03 ± 4.90 21.55 ± 14.72 0.15 ± 0.03 0.53 ± 0.23 28.24 ± 11.10 1.12 ± 0.2 2.19 ± 1.16 0.02 ± 0 Eri-0 12.86 ± 3.96 23.84 ± 13.33 0.13 ± 0.09 0.68 ± 0.48 20.55 ± 8.45 2.04 ± 0.3 2.24 ± 0.93 0.03 ± 0.04 Est-1 14.85 ± 1.63 36.79 ± 2.44 0.42 ± 0.19 2.01 ± 0.86 32.22 ±12.66 0.44 ± 0.2 6.48 ± 3.7 0.07 ± 0.09 Fei-0 12.82 ± 8.73 52.02 ± 15.32 0.09 ± 0.06 0.73 ± 0.37 62.36 ± 28.64 0.52 ± 0.08 2.77 ± 1.53 0.08 ± 0.13 Fuk-1 13.06 ± 7.87 44.36 ± 47.37 0.13 ± 0.01 0.62 ± 0.11 29.23 ± 27.52 8.09 ± 7.02 1.58 ± 0.62 0.05 ± 0.09 Ga-0 3.40 ± 1.11 19.67 ± 12.65 0.03 -

Phenylpropanoids
Phenylpropanoids The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and mediate plant-pollinator interactions as floral pigments and scent compounds. Concentrations of phenylpropanoids within plants are also altered by changes in resource availability. www.MedChemExpress.com 1 Phenylpropanoids Inhibitors & Modulators (+)-Columbianetin (+)-Columbianetin acetate ((S)-Columbianetin) Cat. No.: HY-N0363 ((S)-Columbianetin acetate) Cat. No.: HY-N0363A (+)-Columbianetin is an isomer of Columbianetin. (S)-Columbianetin acetate is an isomer of Columbianetin is a phytoalexin associated with Columbianetin. Columbianetin is a phytoalexin celery (Apium graveolens) resistance to associated with celery (Apium graveolens) pathogens during storage. Columbianetin exhibits resistance to pathogens during storage. excellent anti-fungal and anti-inflammatory Columbianetin exhibits excellent anti-fungal and activity. anti-inflammatory activity. Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 5 mg, 10 mg, 20 mg Size: 5 mg, 10 mg, 20 mg (+)-Guaiacin (+)-Peusedanol Cat. No.: HY-N2247A Cat. No.: HY-N6063 (+)-Guaiacin is a compound extracted of the bark (+)-Peusedanol is a coumarin isolated from of Machilus wangchiana Chun. (Lauraceae). Peucedanumjaponicum. (+)-Guaiacin shows potent in vitro activities against the release of β-glucuronidase in rat polymorphonuclear leukocytes (PMNs) induced by platelet-activating factor (PAF) . -

Coumarin-Piperazine Derivatives As Biologically Active Compounds
Saudi Pharmaceutical Journal 28 (2020) 220–232 Contents lists available at ScienceDirect Saudi Pharmaceutical Journal journal homepage: www.sciencedirect.com Review Coumarin-piperazine derivatives as biologically active compounds Kinga Ostrowska Department of Organic Chemistry, Faculty of Pharmacy, Medical University of Warsaw, 1 Banacha Str., 02 097 Warsaw, Poland article info abstract Article history: A number of psychiatric disorders, including anxiety, schizophrenia, Parkinson’s disease, depression and Received 3 May 2019 others CNS diseases are known to induce defects in the function of neural pathways sustained by the neu- Accepted 29 November 2019 rotransmitters, like dopamine and serotonin. N-arylpiperazine moiety is important for CNS-activity, par- Available online 7 December 2019 ticularly for serotonergic and dopaminergic activity. In the scientific literature there are many examples of coumarin-piperazine derivatives, particularly with arylpiperazines linked to a coumarin system via an Keywords: alkyl liner, which can modulate serotonin, dopamine and adrenergic receptors. Numerous studies have Coumarin revealed that the inclusion of a piperazine moiety could occasionally provide unexpected improvements Arylpiperazinyl moiety in the bioactivity of various biologically active compounds. The piperazine analogs have been shown to Biological activity have a potent antimicrobial activity and they can also act as BACE-1 inhibitors. On the other hand, arylpiperazines linked to coumarin derivatives have been shown to have antiproliferative activity against leukemia, lung, colon, breast, and prostate tumors. Recently, it has been reported that coumarin- piperazine derivatives exhibit a Fneuroprotective effect by their antioxidant and anti-inflammatory activ- ities and they also show activity as acetylcholinesterase inhibitors and antifilarial activity. In this work we provide a summary of the latest advances in coumarin-related chemistry relevant for biological activity. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Biological Potentials of Hymecromone-Based Derivatives: a Systematic Review
Sys Rev Pharm 2020;11(11):438-452 BA miulotifacleotedgreviewcjaourlnalpin tohe ftieled ofnphtarmiacyls of Hymecromone-based derivatives: A systematic review *, PYhaassrmeraFceauktrici aMl uCshteamfaistNryooDreapTarhtammenetr, ACbodllueglaezoizf Pharmacy, Mosul University, Nineveh, Iraq. Corresponding Author: [email protected] ABSTRACT Keywords: Coumarin-based derivatives occupy a prominent position in many fields Hymecromone, Antimicrobial, Antioxidant, Anti-inflammatory, related to medicine and industry. This can be attributed to their multilateral Canotritruemsopro, anndtievnircael, cardio protective. biological activities and diversity of chemical features. Among coumarin-based Yasser Fakri Mustafa derivatives, hymecromone (7-hydroxy-4-methylcoumarin) has attracted great : attention from the medicinal chemists. In addition to its ease preparation, this synthetic coumarin, which is known chemically as 7-hydroxy-4-methyl-2H- Pharmaceutical Chemistry Department, College of Pharmacy, Mosul chromen-2-one, possesses a phenolic hydroxyl group that considers as one of University, Nineveh, Iraq. the most derivatiable functional groups. In the literature, many scientific [email protected] papers described the synthesis and biological activities of hymecromone- *Corresponding author: Yasser Fakri Mustafa email-address: based derivatives. This systematic review focused on the synthesis of these derivatives and description of their biological potentials, especially those related to the antimicrobial, antioxidant, antitumor, -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Scopoletin 8-Hydroxylase a Novel Enzyme Involved in Coumarin Biosynthesis and Iron-Deficiency Responses in Arabidopsis
Scopoletin 8-hydroxylase a novel enzyme involved in coumarin biosynthesis and iron-deficiency responses in Arabidopsis Siwinska, J., Siatkowska, K., Olry, A., Grosjean, J., Hehn, A., Bourgaud, F., Meharg, A. A., Carey, M., Lojkowska, E., & Ihnatowicz, A. (2018). Scopoletin 8-hydroxylase a novel enzyme involved in coumarin biosynthesis and iron-deficiency responses in Arabidopsis. Journal of experimental botany, 69(7), 1735-1748. https://doi.org/10.1093/jxb/ery005 Published in: Journal of experimental botany Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights Copyright the authors 2018. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected].