Basic Adult and Larval Morphology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Moths Light a Way? by John Pickering, Tori Staples and Rebecca Walcott
SOUTHERN LEPIDOPTERISTS NEWS VOLUME 38 NO4. (2016), PG. 331 SAVE ALL SPECIES – MOTHS LIGHT A WAY? BY JOHN PICKERING, TORI STAPLES AND REBECCA WALCOTT Abstract -- What would it take to save all species from snakes, and stinging insects, they pose no health risk. extinction? A new initiative, Save all species, plans to Moths are an exceedingly species-rich group, for which answer this question and provide the tools we need to do the diversity at a terrestrial site will typically exceed any so by 2050. Here we consider the merits and problems other taxon except for beetles. Because moth larvae are associated with inventorying moths to help decide which restricted in their diet to specific host taxa, differences in terrestrial areas to protect. We compare the the assemblages of resident moth species could reflect scientifically-described moth fauna of the British Isles differences across sites in plants and other hosts. If which, with 2,441 species, is taxonomically complete, that’s true, we may be able to use moth inventories as with 11,806 described species from North America north efficient proxies to compare surrounding plant of Mexico, the fauna of which is not fully described. As communities. a percentage of the described moth fauna, there are fewer “macro” moths (Geometroidea, Drepanoidea, Inventorying moths presents challenges, notably, Noctuoidea, Bombycoidea, Lasiocampidae) in the sampling smaller species, describing thousands of British Isles (34.9%) than those known for the United species new to science, and identifying specimens States and Canada (46.1%). We present data on 1,254 accurately. Our experience is that we can identify 99% species for an intensively-studied site in Clarke County, of moths from digital images to species, species-groups, Georgia and consider whether species in the British Isles which contain species of similar appearance, or are generally smaller than ones in Georgia. -

SYSTEMATICS of the MEGADIVERSE SUPERFAMILY GELECHIOIDEA (INSECTA: LEPIDOPTEA) DISSERTATION Presented in Partial Fulfillment of T
SYSTEMATICS OF THE MEGADIVERSE SUPERFAMILY GELECHIOIDEA (INSECTA: LEPIDOPTEA) DISSERTATION Presented in Partial Fulfillment of the Requirements for The Degree of Doctor of Philosophy in the Graduate School of The Ohio State University By Sibyl Rae Bucheli, M.S. ***** The Ohio State University 2005 Dissertation Committee: Approved by Dr. John W. Wenzel, Advisor Dr. Daniel Herms Dr. Hans Klompen _________________________________ Dr. Steven C. Passoa Advisor Graduate Program in Entomology ABSTRACT The phylogenetics, systematics, taxonomy, and biology of Gelechioidea (Insecta: Lepidoptera) are investigated. This superfamily is probably the second largest in all of Lepidoptera, and it remains one of the least well known. Taxonomy of Gelechioidea has been unstable historically, and definitions vary at the family and subfamily levels. In Chapters Two and Three, I review the taxonomy of Gelechioidea and characters that have been important, with attention to what characters or terms were used by different authors. I revise the coding of characters that are already in the literature, and provide new data as well. Chapter Four provides the first phylogenetic analysis of Gelechioidea to include molecular data. I combine novel DNA sequence data from Cytochrome oxidase I and II with morphological matrices for exemplar species. The results challenge current concepts of Gelechioidea, suggesting that traditional morphological characters that have united taxa may not be homologous structures and are in need of further investigation. Resolution of this problem will require more detailed analysis and more thorough characterization of certain lineages. To begin this task, I conduct in Chapter Five an in- depth study of morphological evolution, host-plant selection, and geographical distribution of a medium-sized genus Depressaria Haworth (Depressariinae), larvae of ii which generally feed on plants in the families Asteraceae and Apiaceae. -
A New Macrolepidopteran Moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican Amber
ZooKeys 965: 73–84 (2020) A peer-reviewed open-access journal doi: 10.3897/zookeys.965.54461 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A new macrolepidopteran moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican amber Weiting Zhang1,2, Chungkun Shih3,4, YuHong Shih5, Dong Ren3 1 Hebei GEO University, 136 Huaiandonglu, Shijiazhuang 050031, China 2 State Key Laboratory of Pal- aeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, CAS, Nanjing 210008, China 3 College of Life Sciences and Academy for Multidisciplinary Studies, Capital Normal University, 105 Xisan- huanbeilu, Haidian District, Beijing 100048, China 4 Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013-7012, USA 5 Laboratorio Dominicano De Ambar Y Gemas, Santo Domingo, Dominican Republic Corresponding author: Weiting Zhang ([email protected]) Academic editor: Gunnar Brehm | Received 19 May 2020 | Accepted 12 August 2020 | Published 3 September 2020 http://zoobank.org/05E273DB-B590-42D1-8234-864A787BE6A0 Citation: Zhang W, Shih C, Shih YH, Ren D (2020) A new macrolepidopteran moth (Insecta, Lepidoptera, Geometridae) in Miocene Dominican amber. ZooKeys 965: 73–84. https://doi.org/10.3897/zookeys.965.54461 Abstract A new genus and species of fossil moth, Miogeometrida chunjenshihi Zhang, Shih & Shih, gen. et sp. nov., assigned to Geometridae, is described from Miocene Dominican amber dating from 15–20 Mya. The new genus is characterized by the forewing without a fovea, R1 not anastomosing with Sc, no areole formed by veins R1 and Rs, R1 and Rs1 completely coincident, M2 arising midway between M1 and M3, anal veins 1A and 2A fused for their entire lengths; and the hind wing with Rs running close to Sc + R1 and M2 absent. -

Contributions Toward a Lepidoptera (Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, Thyrididae, Drepanoidea, Geometro
Contributions Toward a Lepidoptera (Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, Thyrididae, Drepanoidea, Geometroidea, Mimalonoidea, Bombycoidea, Sphingoidea, & Noctuoidea) Biodiversity Inventory of the University of Florida Natural Area Teaching Lab Hugo L. Kons Jr. Last Update: June 2001 Abstract A systematic check list of 489 species of Lepidoptera collected in the University of Florida Natural Area Teaching Lab is presented, including 464 species in the superfamilies Drepanoidea, Geometroidea, Mimalonoidea, Bombycoidea, Sphingoidea, and Noctuoidea. Taxa recorded in Psychidae, Yponomeutidae, Sesiidae, Cossidae, Zygaenoidea, and Thyrididae are also included. Moth taxa were collected at ultraviolet lights, bait, introduced Bahiagrass (Paspalum notatum), and by netting specimens. A list of taxa recorded feeding on P. notatum is presented. Introduction The University of Florida Natural Area Teaching Laboratory (NATL) contains 40 acres of natural habitats maintained for scientific research, conservation, and teaching purposes. Habitat types present include hammock, upland pine, disturbed open field, cat tail marsh, and shallow pond. An active management plan has been developed for this area, including prescribed burning to restore the upland pine community and establishment of plots to study succession (http://csssrvr.entnem.ufl.edu/~walker/natl.htm). The site is a popular collecting locality for student and scientific collections. The author has done extensive collecting and field work at NATL, and two previous reports have resulted from this work, including: a biodiversity inventory of the butterflies (Lepidoptera: Hesperioidea & Papilionoidea) of NATL (Kons 1999), and an ecological study of Hermeuptychia hermes (F.) and Megisto cymela (Cram.) in NATL habitats (Kons 1998). Other workers have posted NATL check lists for Ichneumonidae, Sphecidae, Tettigoniidae, and Gryllidae (http://csssrvr.entnem.ufl.edu/~walker/insect.htm). -

Phylogeny and Evolution of Lepidoptera
EN62CH15-Mitter ARI 5 November 2016 12:1 I Review in Advance first posted online V E W E on November 16, 2016. (Changes may R S still occur before final publication online and in print.) I E N C N A D V A Phylogeny and Evolution of Lepidoptera Charles Mitter,1,∗ Donald R. Davis,2 and Michael P. Cummings3 1Department of Entomology, University of Maryland, College Park, Maryland 20742; email: [email protected] 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20560 3Laboratory of Molecular Evolution, Center for Bioinformatics and Computational Biology, University of Maryland, College Park, Maryland 20742 Annu. Rev. Entomol. 2017. 62:265–83 Keywords Annu. Rev. Entomol. 2017.62. Downloaded from www.annualreviews.org The Annual Review of Entomology is online at Hexapoda, insect, systematics, classification, butterfly, moth, molecular ento.annualreviews.org systematics This article’s doi: Access provided by University of Maryland - College Park on 11/20/16. For personal use only. 10.1146/annurev-ento-031616-035125 Abstract Copyright c 2017 by Annual Reviews. Until recently, deep-level phylogeny in Lepidoptera, the largest single ra- All rights reserved diation of plant-feeding insects, was very poorly understood. Over the past ∗ Corresponding author two decades, building on a preceding era of morphological cladistic stud- ies, molecular data have yielded robust initial estimates of relationships both within and among the ∼43 superfamilies, with unsolved problems now yield- ing to much larger data sets from high-throughput sequencing. Here we summarize progress on lepidopteran phylogeny since 1975, emphasizing the superfamily level, and discuss some resulting advances in our understanding of lepidopteran evolution. -

MOTHS and BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed Distributional Information Has Been J.D
MOTHS AND BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed distributional information has been J.D. Lafontaine published for only a few groups of Lepidoptera in western Biological Resources Program, Agriculture and Agri-food Canada. Scott (1986) gives good distribution maps for Canada butterflies in North America but these are generalized shade Central Experimental Farm Ottawa, Ontario K1A 0C6 maps that give no detail within the Montane Cordillera Ecozone. A series of memoirs on the Inchworms (family and Geometridae) of Canada by McGuffin (1967, 1972, 1977, 1981, 1987) and Bolte (1990) cover about 3/4 of the Canadian J.T. Troubridge fauna and include dot maps for most species. A long term project on the “Forest Lepidoptera of Canada” resulted in a Pacific Agri-Food Research Centre (Agassiz) four volume series on Lepidoptera that feed on trees in Agriculture and Agri-Food Canada Canada and these also give dot maps for most species Box 1000, Agassiz, B.C. V0M 1A0 (McGugan, 1958; Prentice, 1962, 1963, 1965). Dot maps for three groups of Cutworm Moths (Family Noctuidae): the subfamily Plusiinae (Lafontaine and Poole, 1991), the subfamilies Cuculliinae and Psaphidinae (Poole, 1995), and ABSTRACT the tribe Noctuini (subfamily Noctuinae) (Lafontaine, 1998) have also been published. Most fascicles in The Moths of The Montane Cordillera Ecozone of British Columbia America North of Mexico series (e.g. Ferguson, 1971-72, and southwestern Alberta supports a diverse fauna with over 1978; Franclemont, 1973; Hodges, 1971, 1986; Lafontaine, 2,000 species of butterflies and moths (Order Lepidoptera) 1987; Munroe, 1972-74, 1976; Neunzig, 1986, 1990, 1997) recorded to date. -

Lepidoptera: Noctuoidea: Erebidae) and Its Phylogenetic Implications
EUROPEAN JOURNAL OF ENTOMOLOGYENTOMOLOGY ISSN (online): 1802-8829 Eur. J. Entomol. 113: 558–570, 2016 http://www.eje.cz doi: 10.14411/eje.2016.076 ORIGINAL ARTICLE Characterization of the complete mitochondrial genome of Spilarctia robusta (Lepidoptera: Noctuoidea: Erebidae) and its phylogenetic implications YU SUN, SEN TIAN, CEN QIAN, YU-XUAN SUN, MUHAMMAD N. ABBAS, SAIMA KAUSAR, LEI WANG, GUOQING WEI, BAO-JIAN ZHU * and CHAO-LIANG LIU * College of Life Sciences, Anhui Agricultural University, 130 Changjiang West Road, Hefei, 230036, China; e-mails: [email protected] (Y. Sun), [email protected] (S. Tian), [email protected] (C. Qian), [email protected] (Y.-X. Sun), [email protected] (M.-N. Abbas), [email protected] (S. Kausar), [email protected] (L. Wang), [email protected] (G.-Q. Wei), [email protected] (B.-J. Zhu), [email protected] (C.-L. Liu) Key words. Lepidoptera, Noctuoidea, Erebidae, Spilarctia robusta, phylogenetic analyses, mitogenome, evolution, gene rearrangement Abstract. The complete mitochondrial genome (mitogenome) of Spilarctia robusta (Lepidoptera: Noctuoidea: Erebidae) was se- quenced and analyzed. The circular mitogenome is made up of 15,447 base pairs (bp). It contains a set of 37 genes, with the gene complement and order similar to that of other lepidopterans. The 12 protein coding genes (PCGs) have a typical mitochondrial start codon (ATN codons), whereas cytochrome c oxidase subunit 1 (cox1) gene utilizes unusually the CAG codon as documented for other lepidopteran mitogenomes. Four of the 13 PCGs have incomplete termination codons, the cox1, nad4 and nad6 with a single T, but cox2 has TA. It comprises six major intergenic spacers, with the exception of the A+T-rich region, spanning at least 10 bp in the mitogenome. -

Beginner S Guide to Moths of the Midwest Geometers
0LGZHVW5HJLRQ86$ %HJLQQHU V*XLGHWR0RWKVRIWKH0LGZHVW*HRPHWHUV $QJHOOD0RRUHKRXVH ,OOLQRLV1DWXUH3UHVHUYH&RPPLVVLRQ Photos: Angella Moorehouse ([email protected]). Produced by: Angella Moorehouse with the assistance of Alicia Diaz, Field Museum. Identification assistance provided by: multiple sources (inaturalist.org; bugguide.net) )LHOG0XVHXP &&%<1&/LFHQVHGZRUNVDUHIUHHWRXVHVKDUHUHPL[ZLWKDWWULEXWLRQEXWFRPPHUFLDOXVHRIWKHRULJLQDOZRUN LVQRWSHUPLWWHG >ILHOGJXLGHVILHOGPXVHXPRUJ@>@YHUVLRQ $ERXWWKH%(*,11(5¶6027+62)7+(0,':(67*8,'(6 Most photos were taken in west-central and central Illinois; a fewDUH from eastern Iowa and north-central Wisconsin. Nearly all were posted to identification websites: BugGuide.netDQG iNaturalist.org. Identification help was provided by Aaron Hunt, Steve Nanz, John and Jane Balaban, Chris Grinter, Frank Hitchell, Jason Dombroskie, William H. Taft, Jim Wiker,DQGTerry Harrison as well as others contributing to the websites. Attempts were made to obtain expert verifications for all photos to the field identification level, however, there will be errors. Please contact the author with all corrections Additional assistance was provided by longtime Lepidoptera survey partner, Susan Hargrove. The intention of these guides is to provide the means to compare photographs of living specimens of related moths from the Midwest to aid the citizen scientists with identification in the field for Bio Blitz, Moth-ers Day, and other night lighting events. A taxonomic list to all the species featured is provided at the end along with some field identification tips. :(%6,7(63529,',1*,'(17,),&$7,21,1)250$7,21 BugGuide.net LNaturalist.org Mothphotographersgroup.msstate.edu Insectsofiowa.org centralillinoisinsects.org/weblog/resources/ :+,&+027+*8,'(7286( The moths were split into 6 groups for the purposes of creating smaller guides focusing on similar features of 1 or more superfamilies. -

Predatory and Parasitic Lepidoptera: Carnivores Living on Plants
Journal of the Lepidopterists' Society 49(4), 1995, 412-453 PREDATORY AND PARASITIC LEPIDOPTERA: CARNIVORES LIVING ON PLANTS NAOMI E. PIERCE Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts, 02138, USA ABSTRACT. Moths and butterflies whose larvae do not feed on plants represent a decided minority slice of lepidopteran diversity, yet offer insights into the ecology and evolution of feeding habits. This paper summarizes the life histories of the known pred atory and parasitic lepidopteran taxa, focusing in detail on current research in the butterfly family Lycaenidae, a group disproportionately rich in aphytophagous feeders and myr mecophilous habits. More than 99 percent of the 160,000 species of Lepidoptera eat plants (Strong et al. 1984, Common 1990). Plant feeding is generally associated with high rates of evolutionary diversification-while only 9 of the 30 extant orders of insects (Kristensen 1991) feed on plants, these orders contain more than half of the total number of insect species (Ehrlich & Raven 1964, Southwood 1973, Mitter et al. 1988, cf. Labandiera & Sepkoski 1993). Phytophagous species are characterized by specialized diets, with fewer than 10 percent having host ranges of more than three plant families (Bernays 1988, 1989), and butterflies being particularly host plant-specific (e.g., Remington & Pease 1955, Remington 1963, Ehrlich & Raven 1964). This kind of life history specialization and its effects on population structure may have contributed to the diversification of phytophages by promoting population subdivision and isolation (Futuyma & Moreno 1988, Thompson 1994). Many studies have identified selective forces giving rise to differences in niche breadth (Berenbaum 1981, Scriber 1983, Rausher 1983, Denno & McClure 1983, Strong et al. -

Redalyc.An Annotated Checklist of the Noctuoidea of Jordan with Remarks
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Kravchenko, V. D.; Revay, E. E.; Mooser, J.; Ronkay, L.; Witt, T.; Speidel, W.; Müller, G. C. An annotated checklist of the Noctuoidea of Jordan with remarks on ecology, phenology and zoogeography. Part III: Xyleni- nae (Lepidoptera: Noctuidae) SHILAP Revista de Lepidopterología, vol. 43, núm. 170, junio, 2015, pp. 181-188 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45541421002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 181-188 An annotated list Part3 3/6/15 10:29 Página 181 SHILAP Revta. lepid., 43 (170), junio 2015: 181-188 eISSN: 2340-4078 ISSN: 0300-5267 An annotated checklist of the Noctuoidea of Jordan with remarks on ecology, phenology and zoogeography. Part III: Xyleninae (Lepidoptera: Noctuidae) V. D. Kravchenko, E. E. Revay, J. Mooser, L. Ronkay, T. Witt, W. Speidel & G. C. Müller Abstract A complete list of the presently known Xyleninae species of Jordan is presented, and the biogeography, phenology and distribution of the same are discussed. Within a German - Israeli project to monitor the Lepidoptera Fauna of the Levant we record from 1986 to 2010 a total of 84 species, 33 of which (39 %) are new records for the country. In Jordan the Xyleninae are represented by eight tribes, most species belonging to the Xylenini (32) and Caradrini (25).The majority of the species are of an Eremic (27), Mediterranean (24) and Irano-Turanian (16) distribution pattern. -
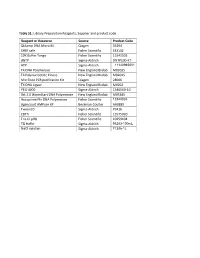
Table S1: Library Preparation Reagents, Supplier and Product Code
Table S1: Library Preparation Reagents, Supplier and product code. Reagent or Resource Source Product Code QIAamp DNA Micro Kit Qiagen 56304 SYBR safe Fisher Scientific S33102 10X Buffer Tango Fisher Scientific 11541505 dNTP Sigma-Aldrich DNTP100-KT ATP Sigma-Aldrich 11140965001 T4 DNA Polymerase New England Biolab M0203S T4 Polynucleotide Kinase New England Biolab M0201S Min Elute PCR purification Kit Qiagen 28006 T4 DNA Ligase New England Biolab M0202 PEG-4000 Sigma-Aldrich 1546569-1G Bst 2.0 WarmStart DNA Polymerase New England Biolab M0538S Accuprime Pfx DNA Polymerase Fisher Scientific 12344024 Agencourt AMPure XP Beckman Coulter A63880 Tween20 Sigma-Aldrich P9416 EDTA Fisher Scientific 15575020 Tris-Cl pH8 Fisher Scientific 10259194 TE buffer Sigma-Aldrich 93283-100mL NaCl solution Sigma-Aldrich 71386-1L Table S2: Complete list of taxa included in the analysis Code Superfamily Family Genus Species NCBI accession Type of data NW_T143 Bombycoidea Apatelodidae Apatelodes pithala SRR1794084 Transcriptome NW_G003 Bombycoidea Bombycidae Bombyx mori GCF_000151625.1 Genome NW_T076 Bombycoidea Saturniidae Actias luna SRR1002974 Transcriptome NW_T134 Bombycoidea Saturniidae Antheraea yamamai SRR1743839 Transcriptome NW_T195 Bombycoidea Saturniidae Attacus atlas SRR1002994 Transcriptome NW_T077 Bombycoidea Saturniidae Eacles imperialis SRR1299435 Transcriptome NW_T131 Bombycoidea Saturniidae Rhodinia newara SRR1743843 Transcriptome NW_T130 Bombycoidea Saturniidae Samia ricini SRR1743841 Transcriptome NW_T078 Bombycoidea Saturniidae Therinia -

Caterpillars on the Foliage of Conifers in the Northeastern United States 1 Life Cycles and Food Plants
INTRODUCTION INTRODUCTION Coniferous forests are important features of the North American landscape. In the Northeast, balsam fir, spruces, or even pines may dominate in the more northern forests. Southward, conifers still may be prevalent, although the pines become increasingly important. In dry, sandy areas, such as Cape Cod of Massachusetts and the Pine Barrens of New Jersey, hard pines abound in forests composed of relatively small trees. Conifers are classic symbols of survival in harsh environments. Forests of conifers provide not only beautiful scenery, but also livelihood for people. Coniferous trees are a major source of lumber for the building industry. Their wood can be processed to make paper, packing material, wood chips, fence posts, and other products. Certain conifers are cultivated for landscape plants and, of course, Christmas trees. Trees of coniferous forests also supply shelter or food for many species of vertebrates, invertebrates, and even plants. Insects that call these forests home far outnumber other animals and plants. Because coniferous forests tend to be dominated by one to a few species of trees, they are especially susceptible to injury during outbreaks of insects such as the spruce budworm, Choristoneura fumiferana, the fall hemlock looper, Lambdina fiscellaria fiscellaria, or the pitch pine looper, Lambdina pellucidaria. Trees that are defoliated by insects suffer reduced growth and sometimes even death. Trees stressed by defoliation, drought, or mechanical injury, are generally more susceptible to attack by wood-boring beetles, diseases, and other organisms. These secondary pests also may kill trees. Stress or tree death can have a negative economic impact upon forest industries.