NEUTRAL RED Powder Dye, C.I. 50040
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solarbio Science & Technology Co., Ltd Tel: 010-56371207 Solarbio Fax: 010-56371281/82
Beijing Solarbio Science & Technology Co., Ltd Tel: 010-56371207 Fax: 010-56371281/82 Solarbio Http://www.solarbio.cn Neutral Red CAS Number: 553-24-2 Storage Temperature: 2-8 °C Product Description : Appearance: Fine dark green-black powder Molecular Formula: C15H17ClN4 Molecular Weight: 288.78 Synonyms: toluylene red, basic red 5 Neutral Red is a weak cationic azine dye that is used extensively as a nuclear stain in a variety of biological stain applications. It is a pH indicator as well, changing color from red to yellow over the pH range 6.8-8.0. It is also incorporated into bacteriological growth media. This product is often used for supravital staining of fresh peripheral blood. It can also be used for staining Nissl granules of neuroglial cells. However, this stain is not as permanent as another dye, Cresyl Violet acetate, for this application. Buffered 0.5% Neutral Red solutions are used as a counterstain for Naphthol AS acetate esterase, peroxidase and iron stains. Solutions can also be used to stain plankton for viability. Using 1 part Neutral Red to 10,000 parts sea water, dead cells were stained red and live cells remained unchanged. In addition, aqueous solutions of Neutral Red (0.1% in saline, pH 6.5) can be used as a fluorescent stain for lipids. Lipids will fluoresce blue-geen or yellow, depending on their composition. It has been used also as a Twort's stain for parasites in combination with Light Green SF, as a general histological stain for embryonic tissue in combination with Janus green,and for demostrating hydrolysis of fats. -

STUDIES of MITOCHONDRIAL STAINING with PINACYANOLE, EMPLOYI}.TG YOSHIDA Ascittss SARCOMA CEI-L
247 STUDIES OF MITOCHONDRIAL STAINING WITH PINACYANOLE, EMPLOYI}.TG YOSHIDA ASCITtsS SARCOMA CEI-L KryosARU TexrrAwA, Kyoreno Aen, Krsuro Kero, Tnnuo Yosnloa AND Kyorcnr MesurANr Department of Internal Meclictne, I(omatsujima RerI Cross Hospital (Chief : Dr. Riyoharu Takikau)a) 7st Departntent of Internal Medicine, Nagoya Uniuersity School of Medicine (Director : Prof . Susumu Hibino) Because of potent activities of respiratory enzymes found in isolated mito- chondria, morphological changes in mitochondria have again attracted attention as indicative of cell's functional potentialities. Mitochondria in the cell can be visualized by employing, 1) Altmann's stain- ing method or Heidenhain's iron hematoxylin stain on fixed preparations, 2) the supravital staining method using Janus green, and recently 3) the supravital observation by means of the phase contrast microscope. Among these, the supravital method with Janus green is widely employed because of its simplicity and high specificity. But this Janus green method is not free from faults : namely difficulty in differentiating the types of cells and quick fading of the stained mitochondria. In 1936 Hetheringtonl) introduced a dyestuff named pinacyanole into the su- pravital staining method of mitochondria, and this method has been investigated by J. L. Schwind,2) showing that nuclei are stained supravitally and the types of cells are easily differentiated, stainability of neutral red vacuoleg is not dis- turbed and the colored mitochondria do not fade away for several hours. This pinacyanole (Consolidated Midland Corporation) and vital neutral red have been obtained lately, and we are discussing the usefulness of the former dyestuff in the study of mitochondria, comparing it with the above-mentioned various mitochondrial methods, and the nature of its staining mechanism. -

||||||||||||III USO0575109A United States Patent (19) 11) Patent Number: 5,175,109 Sakata Et Al
||||||||||||III USO0575109A United States Patent (19) 11) Patent Number: 5,175,109 Sakata et al. (45) Date of Patent: " Dec. 29, 1992 54 REAGENT FOR CLASSIFYING 4,666.82 5/1987 Wiedemann et al. ................. 430/78 LEUKOCYTES BY FLOW CYTOMETRY 4,751,179 6/1988 Ledis et al. ........... ... 424/3 X 4,751,188 6/1988 Valet ............. 436/10 X 4.760.006 7/1988 Pawlowski ............................ 430/78 75) Inventors: Takashi Sakata; Tomoyuki Kuroda, both of Kakogawa, Japan 4.882,284 1 1/1989 Kirchanski et al. .. ... 436/63 73 Assignee: Toa Medical Electronics Co., Ltd., 4,933,293 6/1990 Kuroda et al. ........................ 436/63 Kobe, Japan FOREIGN PATENT DOCUMENTS *) Notice: The portion of the term of this patent O086951 8/1983 European Pat. Off. subsequent to Jun. 12, 2007 has been 1560729 2/1980 France . disclaimed. 55-18860 5/1980 Japan . (21) Appl. No.: 663,090 OTHER PUBLICATIONS Kamentsky, Blood Cells, 6, 121-140 (1980). (22 Filed: Feb. 28, 1991 Shapiro et al., J. Histochem. Cytochem., 24, 396-41 1, Related U.S. Application Data (1976). Shapiro et al., J. Histochem. Cytochem., 25, 976–989 63 Continuation of Ser. No. 91.663, Sep. 1, 1987, aban (1977). doned. Colour Index, vol. 4, published by The Society of Dyers (30) Foreign Application Priority Data and Colourists, pp. 4417-4459 (1971). Sep. 10, 1986 JP Japan ................................ 6-21376 Steinkamp, "Flow Cytometry," Rey. Sci. Instrum... pp. Nov. 27, 1986 JP Japan ................................ 6.-28.2697 1375-1400 (1974). W. Groner & D. Tycko, "Characterizing Blooc Cells 51) Int. Cl. .............................................. C09K11/06 52) U.S. -

STAINING TECHNIQUES Staining Is an Auxiliary Technique Used in Microscopy to Enhance Contrast in the Microscopic Image
STAINING TECHNIQUES Staining is an auxiliary technique used in microscopy to enhance contrast in the microscopic image. Stains or dyes are used in biology and medicine to highlight structures in biological tissues for viewing with microscope. Cell staining is a technique that can be used to better visualize cells and cell components under a microscope. Using different stains, it is possible to stain preferentially certain cell components, such as a nucleus or a cell wall, or the entire cell. Most stains can be used on fixed, or non-living cells, while only some can be used on living cells; some stains can be used on either living or non-living cells. In biochemistry, staining involves adding a class specific (DNA, lipids, proteins or carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescence tagging can serve similar purposes Purposes of Staining The most basic reason that cells are stained is to enhance visualization of the cell or certain cellular components under a microscope. Cells may also be stained to highlight metabolic processes or to differentiate between live and dead cells in a sample. Cells may also be enumerated by staining cells to determine biomass in an environment of interest. Stains may be used to define and examine bulk tissues (e.g. muscle fibers or connective tissues), cell populations (different blood cells) or organelles within individual cells. Biological staining is also used to mark cells in flow cytometry, flag proteins or nucleic acids on gel electrophoresis Staining is not limited to biological materials, it can also be used to study the morphology (form) of other materials e.g. -
Article Text Additional Text Cinr Schulznr Casnr Item Number
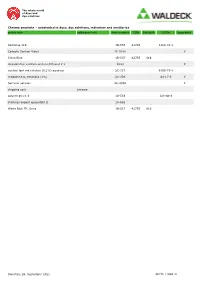
The whole world of dyes and dye solutions Chroma-products – subdivided in dyes, dye solutions, indicators and auxiliaries article text additional text item number CINr SchulzNr CASNr hazardous Acid blue 119 1B-555 42765 1324-76-1 Carbolic Gentian Violet 2E-028K Y China Blue 1B-507 42755 816 discoloration solution acetone/Ethanol 1:1 E333 Y nuclear fast red solution (0,1%) aqueous 2C-337 6409-77-4 rhodamine b, ethanolic (1%) 2C-339 64-17-5 Y Safranin solution 2C-333K Y shipping cost chroma solvent green 3 1B-553 128-80-3 staining reagent eppendahl II 1A-652 Water Blue TR, Unna 1B-517 42755 816 Dienstag, 28. September 2021 SEITE 1 VON 21 The whole world of dyes and dye solutions Chroma-products – subdivided in dyes, dye solutions, indicators and auxiliaries article text additional text item number CINr SchulzNr CASNr hazardous dyes Acid Alizarine Blue B 1A-252 16680 1058-92-0 Acid Black 12 B 1A-598 20470 299 1064-48-8 acid brilliant flavine 7g 1F-562 61968-07-8 Acid Fuchsine-Orange 1F-347 Acid Green G 1B-215 42095 765 5141-20-8 Acid Rhodamine 1A-004 45100 863 3520-42-1 Acridine Orange 3 R zinc chloride double salt 1B-307 46005 10127-02-3 Acridine Yellow 1B-331 46025 135-49-9 Acriflavine 5A-406 46000 906 Y Alcian Green 2 GX 1F-555 Alcian Green 3 BX 1F-551 Alcian Yellow GXS 1F-597 12840 61968-76-1 Alizarine Blue B 1A-246 16680 Alizarine Brilliant Violet R 1B-077 60730 1196 4430-18-6 Alizarine Carmine 1F-581 58005 1145 130-22-3 Alizarine Pure 1A-020 58000 1141 72-48-0 Alizarine Purple RS 1B-079 60730 1196 4430-18-6 Alizarine Red S 1F-583 58005 -

THE COLOUR INDEX (CI) and HISTOLOGICAL STAINS Sir, The
432 Letters to the Editors 3 All equipment including refrigeration and air-conditioning units are individually connected to a solid-state, over/under-voltage cut-out with a user-definable time delay of up to JO min. The ability to individually pre-set the turn-on delay prevents simultaneous activation of all equipment when the power is restored. This prevents over-loading of the power line. In addition, refrigeration and air-conditioning equipment requires a minimum turn-on delay of 3 min to allow the refrigerant to stabilize, thus avoiding damage to the compressor. Visual indication of tripping is provided on each of the units. The prototype, built by one of us, has been functioning satisfactorily for the past 6 years. All the post-prototype units were assembled in the centre by Mr S Charles, a member of the staff, who is self-taught electronics enthusiast. The electronic components used are available locally. 4 The mains supply is monitored by the duty electrician and in the event of a black-out or prolonged brown-out, generators are activated manually. Automatic start-up systems, though available, were not incorporated since there is no requirement for emergency power. Anyone interested in detailed information about the above-mentioned devices is welcome to correspond with us. Schieffelin Leprosy Research J DEVASUNDARAM & A CARIAPPA & Training Centre, Karigiri-632 106, Tamil Nadu, India. VISUAL AIDS; LABELS AND DIAGRAMS TO AID COMPLIANCE Sir, I was interested to read in the December 1986 issue of Leprosy Review (page 373) under ' Leprosy Control and Field Work', about the use of labels and diagrams to aid compliance, from Dr D J Morton and colleagues in the University of Zimbabwe, Africa. -

Special Stains Iron/Hemosiderin Prussian Blue
Special stains Iron/Hemosiderin Prussian blue Lipids Sudan stain (Sudan II, Sudan III, Sudan IV, Oil Red O, Sudan Black B) Carbohydrates Periodic acid-Schiff stain Amyloid Congo red Gram staining (Methyl violet/Gentian violet, Safranin) · Ziehl-Neelsen Bacteria stain/acid-fast (Carbol fuchsin/Fuchsine, Methylene blue) · Auramine- rhodamine stain (Auramine O, Rhodamine B) trichrome stain: Masson's trichrome stain/Lillie's trichrome (Light Green SF yellowish, Biebrich scarlet, Phosphomolybdic acid, Fast Green Connective tissue FCF) Van Gieson's stain H&E stain (Haematoxylin, Eosin Y) · Silver stain (Gömöri methenamine Other silver stain, Warthin–Starry stain) · Methyl blue · Wright's stain · Giemsa stain · Gömöri trichrome stain · Neutral red · Janus Green B Hematoxylin + Eosin (H & E ) הצביעה השגרתית המבוצעת בחתכי רקמה המטוקסילין – צבע בסיסי המתחבר לחומצות הגרעין אאוזין – צבע חומצי המתחבר לקצה הבסיסי של החלבונים בציטופלסמה Pas stain Demonstrate : • Glycogen • Basement membranes • Neutral mucosubstance The GMS Staining Kit is used to demonstrate polysaccharides in the cell walls of fungi and other organisms. This stain is primarily used to distinguish pathogenic fungi such as Aspergillus and Blastomyces and other opportunistic organisms such as Pneumocystis carinii Giemsa Stain The Giemsa is used to differentiate leukocytes in bone marrow and other hematopoietic tissue (lymph nodes) as well as some microorganisms (Helicobacter pylori). The Elastic Staining Kit is used to demonstrate elastic fibers in tissue sections The Mucicarmine Staining -

Gram's Stain Pack
Gram’s Stain Pack HS802 Instructions for use v3 12.01.2017 Product identity (name) Intended Use HS802F, HS802NR, HS802S Gram’s Stain Pack IVD, for professional use only. (synonyms: None) CAS-No: N/A Type (s) of material Pack Size 4 x 250ml Air dried heat fixed bacterial smears from culture or tissue sections. The culture can come from a thick Product composition suspension of a liquid culture or a pure colony from a plate suspended in water. 1. HS230 - Crystal Violet 250ml Contains <5% ethanol (CAS-No 64-17-5 ) Suggested Staining Procedure 1, 2 2. HS305X - Gram’s Iodine Diluent 240ml 3. HS305Y - Gram’s Iodine Concentrate 10ml Principle: Contains Potassim iodide The method is named after its inventor, the Danish <20% (CAS-No 7681-11-0) scientist Hans Christian Gram (1853–1938). Gram staining differentiates bacteria by the chemi- 4. HS310 - Gram’s Decolouriser 250ml cal and physical properties of their cell walls by de- Contains: 75% acetone (CAS-No 67-64-1); tecting peptidoglycan, which is present in a thick 25% Iso-Propanol (CAS-No 67-63-0) layer in Gram-positive bacteria. In the Gram stain, Gram-positive bacteria retain the crystal violet dye, while the counterstain (Safranin, Carbol fuchsin or Neutral red ) added after the crystal violet gives all Gram-negative bacteria a red or pink colouring. 5. i) HS160 - Carbol Fuchsin (Counterstain) 250ml Contains: <0.1% methanol (CAS-No 67-56-1); <1% phenol (CAS No 108-95-2) Handling and treatment before staining: Prepare thin smears using a loopful of a pure cul- ture on grease free slides. -

Finger Mark Development Techniques Within Scope of ISO 17025, Sections 1
Fingerprint Source Book – Chapter 3: Finger mark development techniques within scope of ISO 17025 Chapter 3: Finger mark development techniques within scope of ISO 17025 3.1 Acid dyes (acid black 1, acid violet 17, acid yellow 7) 1. History 1.1 Fingerprints may deposited in a number of contaminants at crime scenes, and of all these blood is the most commonly observed This is possibly because, when present even in small quantities, it is easily seen as it strongly absorbs light throughout the visible spectrum. However, when present in minute amounts, or on dark, patterned or multicoloured confusing backgrounds, the blood may require enhancement to make it more useful for evidential purposes. Additionally, proof that a stain is actually blood rather than an innocuous substance may be important in assessing guilt or innocence, and may even be a matter of life or death in some cases. 1.2 The history of proving the presence of blood evidence in forensic investigation dates back over 150 years using chemical means, and further still when microscopical methods are considered. Anton van Leeuwenhoek was said to be the first person to describe and illustrate blood cells in the latter part of the 17th century, although this is disputed. 1.3 The earliest tests for blood were of two types, both relying on the presence of the haem group present in the red blood cells. The early tests included those that reacted with haem to produce crystals and those that relied on its catalytic nature. More recently (1999) a third test relying on antibodies has been introduced. -

Neutral Red Gurr for Microscopical Staining (C.I
In Vitro Diagnostic Medical Device For professional use only Neutral red Gurr for microscopical staining (C.I. 50040) Cat. No Pack Type Pack Size 2. Janus green B solution 340564A Glass Bottle 25 g Dissolve 0.25 g Janus green B in 25 ml distilled water 340565B Glass Bottle 100 g and filter. 3. Staining solution Composition Mix 25 ml solution 1 and 0.25 ml solution 2. C.I. 50040 C15H17ClN4 Sample material and preparation M = 288.78 g/mol For professional use only. Frech capillary blood, collected by pricking the skin Intended Use(s) In order to avoid errors, the staining process must be carried Neutral red can be used for supravital staining (a stain out by qualified personnel. introduced in living tissue that has been removed from the National guidelines for work safety and quality assurance body, but before cessation of the chemical life of the cells) must be followed. of blood Microscopes equipped according to the standard must be It´s used for the initial evaluation of blood components used. Evaluate the result by comparing it to what would be the age All samples must be clearly labelled. related normal values Review of the samples helps in determining the need for ancillary studies. Procedure An initial review ot the patient´s clinical background is Supravital staining necessary to use in conjunction with the result of the staining Bring 2μl neutral red-janus green B solution to a glass slide, mix with 10 μl blood. Cover with a cover glass, seal the rims. Samples derived from the human body Check immediately and after 2 h under the microscope. -
BD™ Macconkey II Agar
INSTRUCTIONS FOR USE – READY-TO-USE PLATED MEDIA PA-254025.06 Rev.: Mar 2013 BD MacConkey II Agar INTENDED USE BD MacConkey II Agar is a selective and differential medium for the isolation and differentiation of Enterobacteriaceae and a variety of other Gram negative rods from clinical specimens. PRINCIPLES AND EXPLANATION OF THE PROCEDURE Microbiological method. At the present time, many culture media are available for the isolation, cultivation and identification of Enterobacteriaceae and certain nonfermenters. One of the earliest of these was developed by MacConkey and published in 1900 and 1905.1,2 This formulation was devised in the knowledge that bile salts are precipitated by acids and certain enteric micro-organisms ferment lactose whereas others do not possess this ability. Later on, this medium was modified several times.3,4 MacConkey Agar is only slightly selective since the concentration of bile salts, which inhibits gram-positive micro-organisms, is low in comparison with other enteric plating media. This medium is recommended for use with clinical specimens likely to contain mixed microbial flora, such as urine, respiratory, wound, and others, because it allows a preliminary grouping of enteric and other gram-negative bacteria in lactose fermenters and lactose nonfermenters.5,6 MacConkey Agar is also utilized in the microbiological examination of foods.7 The MacConkey II Agar formulation was designed to improve the inhibition of swarming Proteus species, to achieve more definitive differentiation of lactose fermenters and nonfermenters, and for superior growth of enteric bacteria. In BD MacConkey II Agar, peptones provide nutrients. Crystal violet inhibits Gram positive bacteria, especially enterococci and staphylococci. -
Histology Staining - Reagents
Histology Staining - Reagents Staining reagents, eosin, Thermo Scientific Shandon Gram’s staining solutions, ready to use Instant Eosin 04 LE A range of convenient ready prepared stains, which can be used immediately. • Stable and precipitate free – no Stain concentrates are 10 times concentrated to give a ready to use stain on dilution. filtering Catalogue No Description Volume, mL Price • Produces consistent results P/L500/08 Crystal violet 500 8.35 • pH-adjusted for maximum stain P/L500/15 Crystal violet 1,000 11.58 uptake P/L500/90 Crystal violet 2,000 15.30 • Produces brilliant staining P/L510/08 Gram’s iodine 500 9.38 P/L510/15 Gram’s iodine 1,000 15.43 • Compatible with all alum haematoxylin stains P/L510/90 Gram’s iodine 2,000 22.68 P/L520/08 Gram’s differentiator 500 9.91 • Economical powder, stores P/L520/15 Gram’s differentiator 1,000 16.72 indefinitely P/L520/90 Gram’s differentiator 2,000 25.85 • Available in either alcoholic or P/L530/08 Neutral red 500 9.64 aqueous formation P/L530/15 Neutral red 1,000 14.40 P/L530/90 Neutral red 2,000 18.13 Catalogue No Alt. No Description Pack qty Price P/L540/08 Safranin 500 11.70 SD6765040 6765040 Instant eosin, alcoholic, 6 88.95 P/L540/15 Safranin 1,000 15.56 makes 6L P/L540/90 Safranin 2,000 23.07 SD6765540 6765540 Instant eosin, aqueous, 6 88.95 P/L550/08 Dilute carbol fuchsin 500 8.10 makes 6L P/L550/15 Dilute carbol fuchsin 1,000 12.47 P/L550/90 Dilute carbol fuchsin 2,000 17.36 P/L560/08 Lugol’s iodine 500 10.94 Staining reagents, eosin, Thermo Scientific Shandon P/L560/15 Lugol’s iodine 1,000 18.25 Eosin Y P/L560/90 Lugol’s iodine 2,000 32.50 LE Staining reagent, haematoxylin, Thermo Scientific Shandon Instant Haematoxylin LE • No mucoid staining • Stains from blue-black to light blue depending on preparation • Protocol – progressive or regressive • Requires filtration depending on formula • Suitable for frozen sections, plastics, immuno histochemistry, special stains and cytology • May be used as a substitute for Harris or Gill stain Catalogue No Alt.