THE COLOUR INDEX (CI) and HISTOLOGICAL STAINS Sir, The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lysochrome Dyes Sudan Dyes, Oil Red Fat Soluble Dyes Used for Biochemical Staining of Triglycerides, Fatty Acids, and Lipoproteins Product Description
FT-N13862 Lysochrome dyes Sudan dyes, Oil red Fat soluble dyes used for biochemical staining of triglycerides, fatty acids, and lipoproteins Product Description Name : Sudan IV Other names: Sudan R, C.I. Solvent Red 24, C.I. 26105, Lipid Crimson, Oil Red, Oil Red BB, Fat Red B, Oil Red IV, Scarlet Red, Scarlet Red N.F, Scarlet Red Scharlach, Scarlet R Catalog Number : N13862, 100g Structure : CAS: [85-83-6] Molecular Weight : MW: 380.45 λabs = 513-529 nm (red); Sol(EtOH): 0.09%abs =513-529nm(red);Sol(EtOH):0.09% S:22/23/24/25 Name : Sudan III Other names: Rouge Sudan ; rouge Ceresin ; CI 26100; CI Solvent Red 23 Catalog Number : 08002A, 25g Structure : CAS:[85-86-9] Molecular Weight : MW: 352.40 λabs = 513-529 nm (red); Sol(EtOH): 0.09%abs =503-510nm(red);Sol(EtOH):0.15% S:24/25 Name : Sudan Black B Other names: Sudan Black; Fat Black HB; Solvent Black 3; C.I. 26150 Catalog Number : 279042, 50g AR7910, 100tests stain for lipids granules Structure : CAS: [4197-25-5] S:22/23/24/25 Molecular Weight : MW: 456.54 λabs = 513-529 nm (red); Sol(EtOH): 0.09%abs=596-605nm(blue-black) Name : Oil Red O Other names: Solvent Red 27, Sudan Red 5B, C.I. 26125 Catalog Number : N13002, 100g Structure : CAS: [1320-06-5 ] Molecular Weight : MW: 408.51 λabs = 513-529 nm (red); Sol(EtOH): 0.09%abs =518(359)nm(red);Sol(EtOH): moderate; Sol(water): Insoluble S:22/23/24/25 Storage: Room temperature (Z) P.1 FT-N13862 Technical information & Directions for use A lysochrome is a fat soluble dye that have high affinity to fats, therefore are used for biochemical staining of triglycerides, fatty acids, and lipoproteins. -

Student Safety Sheets Dyes, Stains & Indicators
Student safety sheets 70 Dyes, stains & indicators Substance Hazard Comment Solid dyes, stains & indicators including: DANGER: May include one or more of the following Acridine orange, Congo Red (Direct dye 28), Crystal violet statements: fatal/toxic if swallowed/in contact (methyl violet, Gentian Violet, Gram’s stain), Ethidium TOXIC HEALTH with skin/ if inhaled; causes severe skin burns & bromide, Malachite green (solvent green 1), Methyl eye damage/ serious eye damage; may cause orange, Nigrosin, Phenolphthalein, Rosaniline, Safranin allergy or asthma symptoms or breathing CORR. IRRIT. difficulties if inhaled; may cause genetic defects/ cancer/damage fertility or the unborn child; causes damages to organs/through prolonged or ENVIRONMENT repeated exposure. Solid dyes, stains & indicators including Alizarin (1,2- WARNING: May include one or more of the dihydroxyanthraquinone), Alizarin Red S, Aluminon (tri- following statements: harmful if swallowed/in ammonium aurine tricarboxylate), Aniline Blue (cotton / contact with skin/if inhaled; causes skin/serious spirit blue), Brilliant yellow, Cresol Red, DCPIP (2,6-dichl- eye irritation; may cause allergic skin reaction; orophenolindophenol, phenolindo-2,6-dichlorophenol, HEALTH suspected of causing genetic PIDCP), Direct Red 23, Disperse Yellow 7, Dithizone (di- defects/cancer/damaging fertility or the unborn phenylthiocarbazone), Eosin (Eosin Y), Eriochrome Black T child; may cause damage to organs/respiratory (Solochrome black), Fluorescein (& disodium salt), Haem- HARMFUL irritation/drowsiness or dizziness/damage to atoxylin, HHSNNA (Patton & Reeder’s indicator), Indigo, organs through prolonged or repeated exposure. Magenta (basic Fuchsin), May-Grunwald stain, Methyl- ene blue, Methyl green, Orcein, Phenol Red, Procion ENVIRON. dyes, Pyronin, Resazurin, Sudan I/II/IV dyes, Sudan black (Solvent Black 3), Thymol blue, Xylene cyanol FF Solid dyes, stains & indicators including Some dyes may contain hazardous impurities and Acid blue 40, Blue dextran, Bromocresol green, many have not been well researched. -

Solarbio Science & Technology Co., Ltd Tel: 010-56371207 Solarbio Fax: 010-56371281/82
Beijing Solarbio Science & Technology Co., Ltd Tel: 010-56371207 Fax: 010-56371281/82 Solarbio Http://www.solarbio.cn Neutral Red CAS Number: 553-24-2 Storage Temperature: 2-8 °C Product Description : Appearance: Fine dark green-black powder Molecular Formula: C15H17ClN4 Molecular Weight: 288.78 Synonyms: toluylene red, basic red 5 Neutral Red is a weak cationic azine dye that is used extensively as a nuclear stain in a variety of biological stain applications. It is a pH indicator as well, changing color from red to yellow over the pH range 6.8-8.0. It is also incorporated into bacteriological growth media. This product is often used for supravital staining of fresh peripheral blood. It can also be used for staining Nissl granules of neuroglial cells. However, this stain is not as permanent as another dye, Cresyl Violet acetate, for this application. Buffered 0.5% Neutral Red solutions are used as a counterstain for Naphthol AS acetate esterase, peroxidase and iron stains. Solutions can also be used to stain plankton for viability. Using 1 part Neutral Red to 10,000 parts sea water, dead cells were stained red and live cells remained unchanged. In addition, aqueous solutions of Neutral Red (0.1% in saline, pH 6.5) can be used as a fluorescent stain for lipids. Lipids will fluoresce blue-geen or yellow, depending on their composition. It has been used also as a Twort's stain for parasites in combination with Light Green SF, as a general histological stain for embryonic tissue in combination with Janus green,and for demostrating hydrolysis of fats. -

STUDIES of MITOCHONDRIAL STAINING with PINACYANOLE, EMPLOYI}.TG YOSHIDA Ascittss SARCOMA CEI-L
247 STUDIES OF MITOCHONDRIAL STAINING WITH PINACYANOLE, EMPLOYI}.TG YOSHIDA ASCITtsS SARCOMA CEI-L KryosARU TexrrAwA, Kyoreno Aen, Krsuro Kero, Tnnuo Yosnloa AND Kyorcnr MesurANr Department of Internal Meclictne, I(omatsujima RerI Cross Hospital (Chief : Dr. Riyoharu Takikau)a) 7st Departntent of Internal Medicine, Nagoya Uniuersity School of Medicine (Director : Prof . Susumu Hibino) Because of potent activities of respiratory enzymes found in isolated mito- chondria, morphological changes in mitochondria have again attracted attention as indicative of cell's functional potentialities. Mitochondria in the cell can be visualized by employing, 1) Altmann's stain- ing method or Heidenhain's iron hematoxylin stain on fixed preparations, 2) the supravital staining method using Janus green, and recently 3) the supravital observation by means of the phase contrast microscope. Among these, the supravital method with Janus green is widely employed because of its simplicity and high specificity. But this Janus green method is not free from faults : namely difficulty in differentiating the types of cells and quick fading of the stained mitochondria. In 1936 Hetheringtonl) introduced a dyestuff named pinacyanole into the su- pravital staining method of mitochondria, and this method has been investigated by J. L. Schwind,2) showing that nuclei are stained supravitally and the types of cells are easily differentiated, stainability of neutral red vacuoleg is not dis- turbed and the colored mitochondria do not fade away for several hours. This pinacyanole (Consolidated Midland Corporation) and vital neutral red have been obtained lately, and we are discussing the usefulness of the former dyestuff in the study of mitochondria, comparing it with the above-mentioned various mitochondrial methods, and the nature of its staining mechanism. -

Fingerprint Source Book Chapter 3 Finger Mark Development
Fingerprint Source Book – Chapter 3: Finger mark development techniques within scope of ISO 17025 3.7 Powder suspensions 1. History 1.1 During the development of the small particle reagent in the late 1970s, many other particulates were investigated as constituents in the formulation, including amorphous carbon and graphite and the oxides of the magnetic elements cobalt and iron [1]. All of these gave good results, but none were as consistent as molybdenum disulphide and therefore were not pursued as systems for operational use. 1.2 In a significant development that appears to have been overlooked at the time, Haque and co-workers developed an alternative ‘small particle suspension’ based on iron oxide (Fe3O4) in 1989, and stated that this gave better results than the molybdenum disulphide-based small particle reagent in terms of sensitivity and contrast [2]. The new formulation was also noted to work on wetted surfaces, and to enhance further marks previously developed by powdering. This formulation does not appear to have entered widespread use for non-porous surfaces and was not developed further. 1.3 In the mid-1990s similar formulations were developed by researchers at the National Identification Centre, Tokyo Metropolitan Police, who were investigating simple methods for developing fingerprints deposited on the adhesive side of tapes [3]. This was noted by an American police officer on secondment in Japan and after experimentation with black powder suspensions he contacted the Lightning Powder Company, which developed the ‘Sticky-Side Powder’ product now sold commercially, consisting of a pre-mixed powder that was blended with Kodak Photoflo surfactant and distilled water. -

||||||||||||III USO0575109A United States Patent (19) 11) Patent Number: 5,175,109 Sakata Et Al
||||||||||||III USO0575109A United States Patent (19) 11) Patent Number: 5,175,109 Sakata et al. (45) Date of Patent: " Dec. 29, 1992 54 REAGENT FOR CLASSIFYING 4,666.82 5/1987 Wiedemann et al. ................. 430/78 LEUKOCYTES BY FLOW CYTOMETRY 4,751,179 6/1988 Ledis et al. ........... ... 424/3 X 4,751,188 6/1988 Valet ............. 436/10 X 4.760.006 7/1988 Pawlowski ............................ 430/78 75) Inventors: Takashi Sakata; Tomoyuki Kuroda, both of Kakogawa, Japan 4.882,284 1 1/1989 Kirchanski et al. .. ... 436/63 73 Assignee: Toa Medical Electronics Co., Ltd., 4,933,293 6/1990 Kuroda et al. ........................ 436/63 Kobe, Japan FOREIGN PATENT DOCUMENTS *) Notice: The portion of the term of this patent O086951 8/1983 European Pat. Off. subsequent to Jun. 12, 2007 has been 1560729 2/1980 France . disclaimed. 55-18860 5/1980 Japan . (21) Appl. No.: 663,090 OTHER PUBLICATIONS Kamentsky, Blood Cells, 6, 121-140 (1980). (22 Filed: Feb. 28, 1991 Shapiro et al., J. Histochem. Cytochem., 24, 396-41 1, Related U.S. Application Data (1976). Shapiro et al., J. Histochem. Cytochem., 25, 976–989 63 Continuation of Ser. No. 91.663, Sep. 1, 1987, aban (1977). doned. Colour Index, vol. 4, published by The Society of Dyers (30) Foreign Application Priority Data and Colourists, pp. 4417-4459 (1971). Sep. 10, 1986 JP Japan ................................ 6-21376 Steinkamp, "Flow Cytometry," Rey. Sci. Instrum... pp. Nov. 27, 1986 JP Japan ................................ 6.-28.2697 1375-1400 (1974). W. Groner & D. Tycko, "Characterizing Blooc Cells 51) Int. Cl. .............................................. C09K11/06 52) U.S. -

STAINING TECHNIQUES Staining Is an Auxiliary Technique Used in Microscopy to Enhance Contrast in the Microscopic Image
STAINING TECHNIQUES Staining is an auxiliary technique used in microscopy to enhance contrast in the microscopic image. Stains or dyes are used in biology and medicine to highlight structures in biological tissues for viewing with microscope. Cell staining is a technique that can be used to better visualize cells and cell components under a microscope. Using different stains, it is possible to stain preferentially certain cell components, such as a nucleus or a cell wall, or the entire cell. Most stains can be used on fixed, or non-living cells, while only some can be used on living cells; some stains can be used on either living or non-living cells. In biochemistry, staining involves adding a class specific (DNA, lipids, proteins or carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescence tagging can serve similar purposes Purposes of Staining The most basic reason that cells are stained is to enhance visualization of the cell or certain cellular components under a microscope. Cells may also be stained to highlight metabolic processes or to differentiate between live and dead cells in a sample. Cells may also be enumerated by staining cells to determine biomass in an environment of interest. Stains may be used to define and examine bulk tissues (e.g. muscle fibers or connective tissues), cell populations (different blood cells) or organelles within individual cells. Biological staining is also used to mark cells in flow cytometry, flag proteins or nucleic acids on gel electrophoresis Staining is not limited to biological materials, it can also be used to study the morphology (form) of other materials e.g. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0259332 A1 Hassan Et Al
US 20140259332A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0259332 A1 Hassan et al. (43) Pub. Date: Sep. 18, 2014 (54) BREACH OR CONTAMINATION (52) U.S. Cl. INDICATING ARTICLE CPC .................................... A62B 17006 (2013.01) USPC 2/457; 2/168; 428/492; 428/521; 428/423.1; (71) Applicant: Ansell Limited, Richmond (AU) 264/255; 156/156 (72) Inventors: Noorman Bin Abu Hassan, Shah Alam (MY); Michael S. Zedalis, Mendham, NJ (US); Dave Narasimhan, (57) ABSTRACT Flemington, NJ (US) (73) Assignee: Ansell Limited, Richmond (AU) Provided among other things is a breach or contamination indicating elastomeric article for indicating a breach or con (21) Appl. No.: 14/204,513 tamination by a selected chemical or group of chemicals, the article having an exterior and interior and comprising: (a) an (22) Filed: Mar 11, 2014 interior elastomeric layer selected to resist permeation by the selected chemical(s), which interior layer is colored; and (b) Related U.S. Application Data a contiguous or dis-contiguous indicating layer comprising (60) Provisional application No. 61/777,307, filed on Mar. solid particles of hydrophobic dye effective to provide 12, 2013. enhanced color on contacting hexane, cyclohexane, Xylene, s ethyl ether, butyl acetate, MIBK, methanol, acetonitrile and Publication Classification acetic acid; wherein the distribution of the indicating layer provides for a visually noticeable color change against the (51) Int. Cl. background of the colored interior elastomeric layer upon a A62B 7/00 (2006.01) breach or contamination event by a selected chemical. 14 16 18 12 Patent Application Publication Sep. -
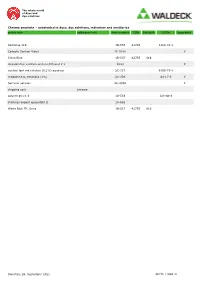
Article Text Additional Text Cinr Schulznr Casnr Item Number
The whole world of dyes and dye solutions Chroma-products – subdivided in dyes, dye solutions, indicators and auxiliaries article text additional text item number CINr SchulzNr CASNr hazardous Acid blue 119 1B-555 42765 1324-76-1 Carbolic Gentian Violet 2E-028K Y China Blue 1B-507 42755 816 discoloration solution acetone/Ethanol 1:1 E333 Y nuclear fast red solution (0,1%) aqueous 2C-337 6409-77-4 rhodamine b, ethanolic (1%) 2C-339 64-17-5 Y Safranin solution 2C-333K Y shipping cost chroma solvent green 3 1B-553 128-80-3 staining reagent eppendahl II 1A-652 Water Blue TR, Unna 1B-517 42755 816 Dienstag, 28. September 2021 SEITE 1 VON 21 The whole world of dyes and dye solutions Chroma-products – subdivided in dyes, dye solutions, indicators and auxiliaries article text additional text item number CINr SchulzNr CASNr hazardous dyes Acid Alizarine Blue B 1A-252 16680 1058-92-0 Acid Black 12 B 1A-598 20470 299 1064-48-8 acid brilliant flavine 7g 1F-562 61968-07-8 Acid Fuchsine-Orange 1F-347 Acid Green G 1B-215 42095 765 5141-20-8 Acid Rhodamine 1A-004 45100 863 3520-42-1 Acridine Orange 3 R zinc chloride double salt 1B-307 46005 10127-02-3 Acridine Yellow 1B-331 46025 135-49-9 Acriflavine 5A-406 46000 906 Y Alcian Green 2 GX 1F-555 Alcian Green 3 BX 1F-551 Alcian Yellow GXS 1F-597 12840 61968-76-1 Alizarine Blue B 1A-246 16680 Alizarine Brilliant Violet R 1B-077 60730 1196 4430-18-6 Alizarine Carmine 1F-581 58005 1145 130-22-3 Alizarine Pure 1A-020 58000 1141 72-48-0 Alizarine Purple RS 1B-079 60730 1196 4430-18-6 Alizarine Red S 1F-583 58005 -

Synthetic Textile Dyes Interacting with Azoreductase
International Journal of ChemTech Research CODEN (USA): IJCRGG ISSN : 0974-4290 Vol.6, No.9, pp 4412-4416, September 2014 RTBCE 2014[12th August 2014] Recent Trends in Biotechnology and Chemical Engineering Involvement of Computational tools towards In Silico remediation - Synthetic textile dyes interacting with Azoreductase 1*S.Sridhar., J.Helan Chandra Department of Biotechnology, Jeppiaar Engineering College, Chennai, India *Corres.author: [email protected]; 9092210251 Abstract: Bacterial Azoreductase plays a critical role in breaking down the azo bond present in the textile azo dyes under aerobic or anaerobic conditions. Technology is delivering a number of bioremediation strategies till date for treating synthetic organics, but these technologies are unlikely to be implemented due to their technical snags in scaling up or during technology transfer to real time scenario. Computational approaches assist us before technology transfer by predicting the nature and toxicity of the interacting ligand with the target receptor protein. Molecular docking is expansively applied in all corners of applied sciences in optimizing the significance and interaction among protein-ligands. Forty azo dyes were selected, based on their wide application in the commercial units. Docking analysis was performed using AutoDock module present inbuilt in PyRx 0.8 version. The top ten dyes with higher affinity towards the target receptor protein is represented in the form of decreasing binding energy with Ponceau MX (-8.61)>Sudan IV (-7.82)> Methyl Yellow (- 7.69)>Citrus Red 2(-7.6)>Scarlet GN(-7.38) > p-diazo violet (-7.19)> Acid Red 14 (-7.18)> Solvent Yellow 3 (-6.93)>Acid Blue 113 (-5.77)> Acid Red 88(-5.03). -

The Effect of Dyes, Pigments and Ionic Liquids on the Properties of Elastomer Com- Posites
The effect of dyes, pigments and ionic liquids onthe properties of elastomer composites Anna Marzec To cite this version: Anna Marzec. The effect of dyes, pigments and ionic liquids on the properties of elastomer com- posites. Polymers. Université Claude Bernard - Lyon I; Uniwersytet lódzki, 2014. English. NNT : 2014LYO10285. tel-01166530v2 HAL Id: tel-01166530 https://tel.archives-ouvertes.fr/tel-01166530v2 Submitted on 23 Jun 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Year 2014 PH.D. THESIS COMPLETED IN “COTUTELLE” THE EFFECT OF DYES, PIGMENTS AND IONIC LIQUIDS ON THE PROPERTIES OF ELASTOMER COMPOSITES presented by MSc. ANNA MARZEC between TECHNICAL UNIVERSITY OF LODZ (POLAND) and UNIVERSITY CLAUDE BERNARD – LYON 1 (FRANCE) For obtaining the degree of Doctor of Philosophy Specialty: Polymers and Composites Materials Defence of the thesis will be held in Lodz, 2 December 2014 Thesis supervisors : Professor MARIAN ZABORSKI (Poland) Doctor GISÈLE BOITEUX (France) JURY ZABORSKI Marian Professor Thesis supervisor BOITEUX Gisèle Doctor Thesis supervisor BEYOU Emmanuel Professor Examiner JESIONOWSKI Teofil Professor Reviewer PIELICHOWSKI Krzysztof Professor Reviewer GAIN Olivier Doctor Thesis co-supervisor Année 2014 Thèse “COTUTELLE” L'EFFET DE COLORANTS, DE PIGMENTS ET LIQUIDES IONIQUES SUR LES PROPRIETES DE COMPOSITES ELASTOMERES présentée par MSc. -

15 Newly Trapped Hares Were Placed in One Unit. Numerous Norway Spruce Bo S Were Stacked for Additional Cover, and Aspen Saplings Were Cut and Supplied R Food
GENERAL NOTES 151 The pen was put into operation on January 15, 1960 an "15 newly trapped hares were placed in one unit. Numerous Norway spruce bo s were stacked for additional cover, and aspen saplings were cut and supplied r food. It was soon found that the nuisance of cutting fresh browse and removing t old, plus the attendant harassment of the hares, could be eliminated by feeding mmercial rabbit pellets. Also, snow accumulated to an extent that required an a II tonal 2 feet of fencing to prevent escape. When hares were later put in the secon unit, spruce and pine boughs were set butts down, three or four together, to form s 11 teepees. The hares readily accepted such shelter and the commercial food pel ts from weather-proof troughs The first lot of 15 hares were held a week for bservation of behavior and con- dition. Then 20 hares were placed in the second nit of the pen. By this time those in the first unit were noticeably quieter and b ter adjusted than the new arrivals. They did, however, continue to "fight" the wire ecause of lessening shelter as the snow deepened and became more impacted. Befo e hares were placed in the third unit, slatted wooden snow fencing was fastened the inside of the poultry wire. This reduced the possibility of eye injury from t wire and increased the protective cover. Snow fencing was later installed in the oth r units and would be desirable even though 1-inch-mesh wire were used. By the end of the winter of 1960 t hares had so chewed the wooden snow fence that some entanglement occurred betw en it and the outside poultry wire.