The Structure of Trichocysts in Paramecium Caudatum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Life: the Science of Biology
Lecture Notebook to accompany SINAUER ASSOCIATES, INC. MACMILLAN Copyright © 2014 Sinauer Associates, Inc. Cover photograph © Alex Mustard/naturepl.com. This document may not be modified or distributed (either electronically or on paper) without the permission of the publisher, with the following exception: Individual users may enter their own notes into this document and may print it for their own personal use. The Origin and Diversification of 0027 Eukaryotes 27.1 A Hypothetical Sequence for the Evolution of the Eukaryotic Cell (Page 550) 1 The protective Cell wall cell wall was lost. DNA 2 Infolding of the plasma membrane added surface area without increasing the cell’s volume. 3 Cytoskeleton (microfilament and microtubules) formed. 4 Internal membranes studded with ribosomes formed. 5 As regions of the infolded plasma membrane enclosed the cell’s DNA, a precursor of a nucleus formed. 6 Microtubules from the cytoskeleton formed the eukaryotic flagellum, 7 Early digestive vacuoles enabling propulsion. evolved into lysosomes using enzymes from the early endoplasmic reticulum. 8 Mitochondria formed through endosymbiosis with a proteobacterium. 9 Endosymbiosis with cyanobacteria led to the development of chloroplasts. Flagellum To add your own notes to any page, use Adobe Reader’s Chloroplast Typewriter feature, accessible via the Typewriter bar at Mitochondrion the top of the window. (Requires Adobe Reader 8 or later. Adobe Reader can be downloaded free of charge from the Nucleus Adobe website: http://get.adobe.com/reader.) LIFE2 The Science of Biology 10E Sadava © 2014 Sinauer Associates, Inc. Sinauer Associates Morales Studio Figure 27.01 05-24-12 Chapter 27 | The Origin and Diversification of Eukaryotes 3 (A) Primary endosymbiosis Eukaryote Cyanobacterium Cyanobacterium outer membrane Peptidoglycan Cyanobacterium inner membrane Host cell nucleus Chloroplast Peptidoglycan has been lost except in glaucophytes. -

Gaits in Paramecium Escape
Transitions between three swimming gaits in Paramecium escape Amandine Hamela, Cathy Fischb, Laurent Combettesc,d, Pascale Dupuis-Williamsb,e, and Charles N. Barouda,1 aLadHyX and Department of Mechanics, Ecole Polytechnique, Centre National de la Recherche Scientifique, 91128 Palaiseau cedex, France; bActions Thématiques Incitatives de Genopole® Centriole and Associated Pathologies, Institut National de la Santé et de la Recherche Médicale Unité-Université d’Evry-Val-d’Essonne Unité U829, Université Evry-Val d'Essonne, Bâtiment Maupertuis, Rue du Père André Jarlan, 91025 Evry, France; cInstitut National de la Santé et de la Recherche Médicale Unité UMRS-757, Bâtiment 443, 91405 Orsay, France; dSignalisation Calcique et Interactions Cellulaires dans le Foie, Université de Paris-Sud, Bâtiment 443, 91405 Orsay, France; and eEcole Supérieure de Physique et de Chimie Industrielles ParisTech, 10 rue Vauquelin, 75005 Paris, France Edited* by Harry L. Swinney, University of Texas at Austin, Austin, TX, and approved March 8, 2011 (received for review November 10, 2010) Paramecium and other protists are able to swim at velocities reach- or in the switching between the different swimming behaviors ing several times their body size per second by beating their cilia (11, 13–17). in an organized fashion. The cilia beat in an asymmetric stroke, Below we show that Paramecium may also use an alternative to which breaks the time reversal symmetry of small scale flows. Here cilia to propel itself away from danger, which is based on tricho- we show that Paramecium uses three different swimming gaits to cyst extrusion. Trichocysts are exocytotic organelles, which are escape from an aggression, applied in the form of a focused laser regularly distributed along the plasma membrane in Paramecium heating. -

Tuberlatum Coatsi Gen. N., Sp. N. (Alveolata, Perkinsozoa), a New
Protist, Vol. 170, 82–103, February 2019 http://www.elsevier.de/protis Published online date 21 December 2018 ORIGINAL PAPER Tuberlatum coatsi gen. n., sp. n. (Alveolata, Perkinsozoa), a New Parasitoid with Short Germ Tubes Infecting Marine Dinoflagellates 1 Boo Seong Jeon, and Myung Gil Park LOHABE, Department of Oceanography, Chonnam National University, Gwangju 61186, Republic of Korea Submitted October 16, 2018; Accepted December 15, 2018 Monitoring Editor: Laure Guillou Perkinsozoa is an exclusively parasitic group within the alveolates and infections have been reported from various organisms, including marine shellfish, marine dinoflagellates, freshwater cryptophytes, and tadpoles. Despite its high abundance and great genetic diversity revealed by recent environmental rDNA sequencing studies, Perkinsozoa biodiversity remains poorly understood. During the intensive samplings in Korean coastal waters during June 2017, a new parasitoid of dinoflagellates was detected and was successfully established in culture. The new parasitoid was most characterized by the pres- ence of two to four dome-shaped, short germ tubes in the sporangium. The opened germ tubes were biconvex lens-shaped in the top view and were characterized by numerous wrinkles around their open- ings. Phylogenetic analyses based on the concatenated SSU and LSU rDNA sequences revealed that the new parasitoid was included in the family Parviluciferaceae, in which all members were comprised of two separate clades, one containing Parvilucifera species (P. infectans, P. corolla, and P. rostrata), and the other containing Dinovorax pyriformis, Snorkelia spp., and the new parasitoid from this study. Based on morphological, ultrastructural, and molecular data, we propose to erect a new genus and species, Tuberlatum coatsi gen. -

Lateral Gene Transfer of Anion-Conducting Channelrhodopsins Between Green Algae and Giant Viruses
bioRxiv preprint doi: https://doi.org/10.1101/2020.04.15.042127; this version posted April 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 5 Lateral gene transfer of anion-conducting channelrhodopsins between green algae and giant viruses Andrey Rozenberg 1,5, Johannes Oppermann 2,5, Jonas Wietek 2,3, Rodrigo Gaston Fernandez Lahore 2, Ruth-Anne Sandaa 4, Gunnar Bratbak 4, Peter Hegemann 2,6, and Oded 10 Béjà 1,6 1Faculty of Biology, Technion - Israel Institute of Technology, Haifa 32000, Israel. 2Institute for Biology, Experimental Biophysics, Humboldt-Universität zu Berlin, Invalidenstraße 42, Berlin 10115, Germany. 3Present address: Department of Neurobiology, Weizmann 15 Institute of Science, Rehovot 7610001, Israel. 4Department of Biological Sciences, University of Bergen, N-5020 Bergen, Norway. 5These authors contributed equally: Andrey Rozenberg, Johannes Oppermann. 6These authors jointly supervised this work: Peter Hegemann, Oded Béjà. e-mail: [email protected] ; [email protected] 20 ABSTRACT Channelrhodopsins (ChRs) are algal light-gated ion channels widely used as optogenetic tools for manipulating neuronal activity 1,2. Four ChR families are currently known. Green algal 3–5 and cryptophyte 6 cation-conducting ChRs (CCRs), cryptophyte anion-conducting ChRs (ACRs) 7, and the MerMAID ChRs 8. Here we 25 report the discovery of a new family of phylogenetically distinct ChRs encoded by marine giant viruses and acquired from their unicellular green algal prasinophyte hosts. -

Chloroplast Structure of the Cryptophyceae
CHLOROPLAST STRUCTURE OF THE CRYPTOPHYCEAE Evidence for Phycobiliproteins within Intrathylakoidal Spaces E . GANTT, M . R . EDWARDS, and L . PROVASOLI From the Radiation Biology Laboratory, Smithsonian Institution, Rockville, Maryland 20852, the Division of Laboratories and Research, New York State Department of Health, Albany, New York 12201, and the Haskins Laboratories, New Haven, Connecticut 06520 ABSTRACT Selective extraction and morphological evidence indicate that the phycobiliproteins in three Cryptophyceaen algae (Chroomonas, Rhodomonas, and Cryptomonas) are contained within intrathylakoidal spaces and are not on the stromal side of the lamellae as in the red and blue-green algae . Furthermore, no discrete phycobilisome-type aggregates have thus far been observed in the Cryptophyceae . Structurally, although not necessarily functionally, this is a radical difference . The width of the intrathylakoidal spaces can vary but is gen- erally about 200-300 A . While the thylakoid membranes are usually closely aligned, grana- type fusions do not occur. In Chroomonas these membranes evidence an extensive periodic display with a spacing on the order of 140-160 A . This periodicity is restricted to the mem- branes and has not been observed in the electron-opaque intrathylakoidal matrix . INTRODUCTION The varied characteristics of the cryptomonads (3), Greenwood in Kirk and Tilney-Bassett (10), are responsible for their indefinite taxonomic and Lucas (13) . The thylakoids have a tendency position (17), but at the same time they enhance to be arranged in pairs, that is, for two of them to their status in evolutionary schemes (1, 4) . Their be closely associated with a 30-50 A space between chloroplast structure is distinct from that of every them . -

Dinophyceae), with Notes on Feeding Behavior and the Description of the Flagellar Base Area of a Planozygote1
J. Phycol. 42, 434–452 (2006) r 2006 Phycological Society of America DOI: 10.1111/j.1529-8817.2006.00195.x ULTRASTRUCTURE AND LSU rDNA-BASED PHYLOGENY OF ESOPTRODINIUM GEMMA (DINOPHYCEAE), WITH NOTES ON FEEDING BEHAVIOR AND THE DESCRIPTION OF THE FLAGELLAR BASE AREA OF A PLANOZYGOTE1 Anto´nio J. Calado,2 Sandra C. Craveiro Departamento de Biologia, Universidade de Aveiro, P-3810-193 Aveiro, Portugal Niels Daugbjerg and Øjvind Moestrup Department of Phycology, Biological Institute, University of Copenhagen, Øster Farimagsgade 2D, DK-1353 Copenhagen K, Denmark A small, freshwater dinoflagellate with an incom- Markov chains; MP, maximum parsimony; NJ, plete cingulum, identified as Esoptrodinium gemma neighbor-joining; PP, posterior probabilities; PSC, Javornicky´ (5Bernardinium bernardinense sensu au- peduncular striated collar; SRC, striated root con- ctt. non sensu Chodat), was maintained in mixed nective (TSR to LMR); TB, transverse basal body; culture and examined using light and serial section TMR, transverse microtubular root; TMRE, TMR TEM. Vegetative flagellate cells, large cells with two extension; TSR, transverse striated root; TSRM, longitudinal flagella (planozygotes), and cysts were transverse striated root microtubule; VR, ventral examined. The cells displayed a red eyespot near ridge the base of the longitudinal flagellum, made of two or three layers of pigment globules not bounded by a membrane. Yellow-green, band-shaped chloro- plasts, bounded by three membranes and contain- The organism used in this study is a naked dino- ing lamella with three thylakoids, were present in flagellate that would be readily identified as Bern- both flagellate cells and cysts. Most cells had food ardinium bernardinense Chodat by recent dinoflagellate vacuoles, containing phagotrophically ingested floras (Starmach 1974, Matvienko and Litvinenko chlamydomonads or chlorelloid green algae; inges- 1977, Popovsky´ and Pfiester 1990). -

Download File
Acta Protozool. (2012) 51: 305–318 http://www.eko.uj.edu.pl/ap ACTA doi:10.4467/16890027AP.12.024.0784 PROTOZOOLOGICA Morphological Description of Telaepolella tubasferens n. g. n. sp., Isolate ATCC© 50593™, a Filose Amoeba in the Gracilipodida, Amoebozoa Daniel J. G. LAHR1,2*, Gabriela M. KUBIK1*, Anastasia L. GANT1, Jessica GRANT1, O. Roger ANDERSON3 and Laura A. KATZ1,2 1Department of Biological Sciences, Smith College, Northampton, MA, USA; 2Program in Organismic and Evolutionary Biology, University of Massachusetts, Amherst, MA, USA; 3Biology, Lamont-Doherty Earth Observatory of Columbia University, Palisades, New York; * D. J. G. Lahr and G. M. Kubik contributed equally to this work Abstract. We describe the amoeboid isolate ATCC© 50593™ as a new taxon, Telaepolella tubasferens n. g. n. sp. This multinucleated amoeba has filose pseudopods and is superficially similar to members of the vampyrellids (Rhizaria) such as Arachnula impatiens Cien- kowski, 1876, which was the original identification upon deposition. However, previous multigene analyses place this taxon within the Gracilipodida Lahr and Katz 2011 in the Amoebozoa. Here, we document the morphology of this organism at multiple life history stages and provide data underlying the description as a new taxon. We demonstrate that T. tubasferens is distinct from Arachnula and other rhizari- ans (Theratromyxa, Leptophrys) in a suite of morphological characters such as general body shape, relative size of pseudopods, distinction of ecto- and endoplasmic regions, and visibility of nuclei in non-stained cells (an important diagnostic character). Although Amoebozoa taxa generally have lobose pseudopods, genera in Gracilipodida such as Flamella and Filamoeba as well as several organisms previously classified as protosteloid amoebae (e.g. -
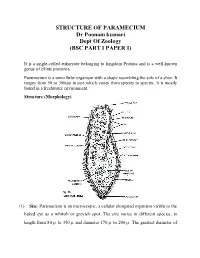
STRUCTURE of PARAMECIUM Dr Poonam Kumari Dept of Zoology (BSC PART I PAPER I)
STRUCTURE OF PARAMECIUM Dr Poonam kumari Dept Of Zoology (BSC PART I PAPER I) It is a single-celled eukaryote belonging to kingdom Protista and is a well-known genus of ciliate protozoa. Paramecium is a unicellular organism with a shape resembling the sole of a shoe. It ranges from 50 to 300um in size which varies from species to species. It is mostly found in a freshwater environment. Structure (Morphology) (1) Size: Paramecium is an microscopic, a cellular elongated organism visible to the baked eye as a whitish or greyish spot. The size varies in different species, in length from 80 to 350 and diameter 170 to 290 . The greatest diameter of the cylindrical body is about two third of its entire length. Usually the individuals of the same species may show minor morphological and physiological differences. (2) Shape: Paramecium is a slipper shaped, cigar shaped, or spindle shaped animalcule. Its shape is usually constant and a symmetrical, because slipper like shape. The body is elongated, blunt and rounded at the anterior end and somewhat pointed of the posterior end. In cross section it is circular with greatest diameter behind the centre of body. The anterior half of the body is slightly twisted. The body is distinguished into an oral or ventral surface and an aboral or dorsal surface. The structure is more complicated due to the development of certain organelles in the acellular body. (3) Oral groove: The ventral surface of body bears a prominent, oblique and shallow depression is called oral groove, it arise from the middle of body and extends to the left side of anterior end. -

Giant Protistan Parasites on the Gills of Cephalopods (Mollusca)
DISEASES OF AQUATIC ORGANISMS Vol. 3: 119-125. 1987 Published December 14 Dis. aquat. Org. Giant protistan parasites on the gills of cephalopods (Mollusca) Norman ~c~ean',F. G. ~ochberg~,George L. shinn3 ' Biology Department, San Diego State University, San Diego, California 92182-0057, USA Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta Del Sol Road, Santa Barbara, California 93105. USA Division of Science, Northeast Missouri State University, Kirksville. Missouri 63501, USA ABSTRACT: Large Protista of unknown taxonomic affinities are described from 3 species of coleoid squids, and are reported from many other species of cephalopods. The white to yellow-orange, ovoid cyst-like parasites are partially embedded within small pockets on the surface of the gills, often in large numbers. Except for a holdfast region on one side of the large end, the surface of the parasite is elaborated into low triangular plates separated by grooves. The parasites are uninucleate; their cytoplasm bears lipid droplets and presumed paraglycogen granules. Trichocysts, present in a layer beneath the cytoplasmic surface, were found by transmission electron microscopy to be of the dino- flagellate type. Further studies are needed to clarify the taxonomic position of these protists. INTRODUCTION epoxy resin (see below). One specimen each of the coleoid squids Abralia trigonura and Histioteuthis dof- Cephalopods harbor a diversity of metazoan and leini were trawled near Oahu, Hawaii, in March, 1980. protozoan parasites (Hochberg 1983). In this study we Gill parasites from the former were fixed in formalin; used light and electron microscopy to characterize a those from the latter were fixed in osmium tetroxide. -

Synchronous Exocytosis in Paramecium Cells. Vi. Ultrastructural Analysis of Membrane Resealing and Retrieval
J.CellSd. 77,1-17(1985) Printed in Great Britain © Company of Biologists Limited 1985 SYNCHRONOUS EXOCYTOSIS IN PARAMECIUM CELLS. VI. ULTRASTRUCTURAL ANALYSIS OF MEMBRANE RESEALING AND RETRIEVAL H. PLATTNER*. R. PAPE, B. HAACKE, K. OLBRICHT, C. WESTPHAL AND H. KERSKEN Faculty of Biology, University ofKonstanz, P.OJi. 5560, D-7750 Konstanz, Federal Republic of Germany SUMMARY After the synchronous induction of exocytosis of secretory organelles (trichocysts) in Paramecium tetraurelia cells the process of membrane resealing and retrieval could be followed under syn- chronous conditions. The characteristic aggregates of membrane intercalated particles (MIPs) contained within the freeze-fractured cell membrane (rings and rosettes) and trichocyst membranes (annulus MIPs), in addition to collar striations on the top of trichocyst membranes, served as endogenous ultrastructural markers. This allowed us to follow the re-arrangement of membrane constituents during and after exocytosis with high temporal and spatial precision. Membrane specificity is maintained to a considerable extent (~ 99-5 %), as judged from the rare occurrence of aberrant resealing (according to freeze-fracture data) and from the rather minute shift of glycocalyx components (according to electron staining experiments) during normal membrane resealing. Coated pits are not involved in membrane retrieval (155 ghosts analysed); since the membrane regions involved in exocytotic fusion are backed by apposed materials, probably proteins, this may restrain membrane constituents from intermixing. Another factor for maintaining membrane specificity is the fact that resealing of the exocytotic opening occurs much more rapidly than in most other systems. The retrieval operates with a half-life of 3 (strain 75) to 9min (K401); the involve- ment of cortical microtubules in the retrieval can be largely excluded, since only two microtubules (of unidentified origin) were seen to approach ghost structures in 4074 cases analysed during this period of intense ghost retrieval. -

Protist Review.Pdf
Protists Termite mound Section 1 Introduction to Protists -!). )DEA Protists form a diverse group of organisms that are subdivided based on their method of obtaining nutrition. Termite colony Section 2 Protozoans— Animal-like Protists -!). )DEA Protozoans are animal-like, heterotrophic protists. Section 3 Algae—Plantlike Protists -!). )DEA Algae are plantlike, autotrophic protists that are the producers for aquatic ecosystems. Section 4 Termites Funguslike Protists SEM Magnification: 17؋ -!). )DEA Funguslike protists obtain their nutrition by absorbing nutrients from dead or decaying organisms. BioFacts • A protist that lives symbiotically Protists in termite gut in the gut of termites helps it LM Magnification: 65؋ digest cellulose found in wood. • The amoeba Amoeba proteus is so small that it can survive in the film of water surrounding particles of soil. • An estimated five million protists can live in one teaspoon of soil. 540 (t)Oliver Meckes/Nicole Ottawa/Photo Researchers, (c)Gerald and Buff Corsi/Visuals Unlimited, (b)Michael Abbey/Photo Researchers , (bkgd)Gerald and Buff Corsi/Visuals Unlimited Start-Up Activities Classify Protists Make this LAUNCH Lab Foldable to help you organize the characteristics of protists. What is a protist? The Kingdom Protista is similar to a drawer or closet in which you keep odds and ends that do not seem to fit any other place. The Kingdom Protista is composed of three groups of organisms that do not fit in any STEP 1 Fold a sheet of notebook paper other kingdom. In this lab, you will observe the three in half vertically. Fold the sheet into thirds. groups of protists. Procedure 1. -

Protistology the Taxonomic Position of Klosteria Bodomorphis Gen. and Sp. Nov. (Kinetoplastida) Based on Ultra Structure And
Protistology 3 (2), 126-135 (2003) Protistology The taxonomic position of Klosteria bodomorphis gen. and sp. nov. (Kinetoplastida) based on ultra- structure and SSU rRNA gene sequence analysis Sergey I. Nikolaev1, Alexander P. Mylnikov2, Cedric Berney3, Jose Fahrni3, Nikolai Petrov1 and Jan Pawlowski3 1 A. N. Belozersky Institute of Physico-Chemical Biology, Department of Evolutionary Biochemistry, Moscow State University, Moscow, Russia 2 Institute for Biology of Inland Water RAS, Borok, Russia 3 Department of Zoology and Animal Biology, University of Geneva, Switzerland Summary A small free-living marine bacteriotrophic flagellate Klosteria bodomorphis gen. and sp. nov. was investigated by electron microscopy and molecular methods. This protist has paraxial rods of typical bodonid structure in the flagella, mastigonemes on the anterior flagellum, two nearly parallel basal bodies and discoid mitochondrial cristae. The flagellar pocket and cytostome/cytopharynx complex are supported by two microtubular roots and reinforced microtubular band (mtr). These features confirm that K. bodomorphis is a bodonid related to Bodo. However, the presence of a layer of dense glycocalyx on the flagella and one battery of cylindrical trichocysts with reticular walls makes it more similar to Rhynchobodo/Phyllomitus, although this flagellate characterized by pankinetoplasty. Phylogenetic analysis using the SSU rRNA gene is congruent with the ultrastructural studies and strongly confirms the close relationship of K. bodomorphis to the genus Rhynchobodo within the order Kinetoplastida. Key words: Bodonidae, Klosteria bodomorphis, evolution, 18S rDNA, phylogeny Introduction of kinetoplastid and related flagellates have been reinvestigated by electron microscopy (Brugerolle et al., Free-living kinetoplastids, especially bodonids 1979; Brugerolle, 1985; Mylnikov, 1986; Mylnikov et al., (Bodonidae Hollande), are an important component of 1998; Elbrächter et al., 1996; Simpson et al., 1997; marine ecosystems.