Equipment Sterilization Disinfection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Theory of Operation: How Parts Cleaning Works
Theory of Operation: How Parts Cleaning Works MART defines the term to clean as to overpower the soils. The MART Parts Washer is a high-impact pressure, high-temperature, industrial water-based cleaning system that uses a combination of the following factors to achieve cleaning results: Power x Temperature x Chemical x Time = Clean The relationship of these variables can be varied in an infinite number of ways to achieve the same level of cleanliness. Your own needs determine the relative value of each variable. Keep in mind that the MART Parts Washer provides one of the highest blasting powers in the cleaning industry, allowing you to reduce the wash-cycle times for your parts to a minimum. Additionally, the high blasting power allows you to operate the washer at lower cleaning temperatures, thus saving energy, and using less chemical than spray washers The exact combination of the factors must be determined for your application, based on the types of soils to be removed, the degree of cleanliness required, the cycle time required, the types of parts to be cleaned, and so on. How the Parts Washer Works The parts washer operates on a timed cycle. The operator places the parts to be cleaned in the washer on the turntable, closes and latches the door, and then starts the timed cleaning cycle. During the cleaning cycle, a high-temperature, high-pressure, water-and-detergent cleaning solution blasts soils from the parts. After the cycle has stopped and the steam has exhausted, the operator removes the cleaned parts. The parts washer utilizes closed loop, waste minimization technology, continuously reusing its cleaning solution and effectively reducing pollution potential. -

The Biomatic® Parts Cleaning System Is…
BIOMATIC® The Environmentally Friendly Bioremediation Parts Washer 1 The Biomatic® Parts Cleaning System Is… • A Bioremediation System. The Graymills Biomatic Parts Cleaning System uses the natural technology of microbial bioremediation to reduce oils, greases and other hydrocarbons introduced into the cleaning system to water and carbon dioxide (CO2). No oil and hydrocarbon buildup means the unit continues to clean parts indefinitely (we recommend cleaning the tank once a year, but the fluid can be put back in for continued use if desired.) • A Waste Minimization System. Talking to your customers about “waste minimization” will get their attention. With the Graymills Biomatic Parts Cleaning System, users will go from monthly dumping of fluid and unit cleanup…to once a year. That’s a “waste minimization” sales story few customers can resist. • A Tough Cleaner. Aqueous based Super Biotene® is pH neutral with a mild, pleasant odor. A mixture of emulsifiers and surfactants, they contain no VOCs, so they’re EPA and air quality friendly! Super Biotene cleans grease and grime off even the dirtiest parts, then the microbes go to work, reducing the hydrocarbon contaminants (oils, greases) to water and CO2. • A Good Investment. Recycling costs are slashed…parts cleaner waste is minimized…the dirtiest parts come clean…and the system is operator friendly. 2 How The Biomatic® Parts Cleaning System Works It is important to understand that the Biomatic® Parts Cleaning System is more than individual products - it is a total cleaning environment. The Biomatic Parts Cleaning System relies on the perfect balance between the Super Biotene® cleaning solution and the microbes introduced into the system. -
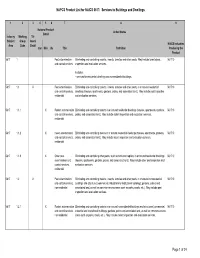
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings 1 2 3 456 7 8 9 National Product United States Detail Industry Working Tri- Subject Group lateral NAICS Industries Area Code Detail Can Méx US Title Definition Producing the Product 5617 1 Pest extermination Eliminating and controlling rodents, insects, termites and other pests. May include termite/pest 561710 and control services inspection and evaluation services. Includes: • services for residential dwellings and nonresidential buildings. 5617 1.1 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around residential 561710 and control services, dwellings (houses, apartments, gardens, patios, and associated land). May include pest inspection residential and evaluation services. 5617 1.1.1 X Rodent extermination Eliminating and controlling rodents in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include rodent inspection and evaluation services. residential 5617 1.1.2 X Insect extermination Eliminating and controlling insects in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include insect inspection and evaluation services. residential 5617 1.1.9 X Other pest Eliminating and controlling other pests. such as birds and reptiles, in or around residential dwellings 561710 extermination and (houses, apartments, gardens, patios, and associated land). May include other pest inspection and control services, evaluation services. residential 5617 1.2 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around nonresidential 561710 and control services, buildings and structures (commercial, industrial and institutional buildings; gardens, patios and nonresidential associated land, as well as common-access areas such as parks, roads, etc.). -

We Take Standards for Hygiene and Cleanliness Very Seriously and Are Taking Additional Steps to Ensure the Safety of Our Guests and Associates
We take standards for hygiene and cleanliness very seriously and are taking additional steps to ensure the safety of our guests and associates. On a daily basis, associates at our hotel, villas and homes, clubhouses, nature center and other public areas are working to ensure that they meet the latest guidance on hygiene and cleaning. Our health and safety measures are designed to address a broad spectrum of viruses, including COVID-19, and include everything from frequent handwashing hygiene and cleaning product specifications to guest room and common area cleaning procedures. Specific steps Kiawah Island Golf Resort is taking include: Associate Health, Safety and Knowledge: Resort associates – and their own health, safety and knowledge – are essential to an effective cleaning program. Here are some ways we’re supporting them: • Hand Hygiene: Proper and frequent handwashing is vital to help combat the spread of viruses. In our daily meetings, our teams are reminded that cleanliness starts with this simple act. It’s important for their health and that of our guests. • Ongoing Training: In addition to training on housekeeping and hygiene protocols, resort associates are also completing enhanced COVID-19 awareness training. • Real Time Information: Kiawah Island Golf Resort continues to coordinate with national, regional, state and local authorities, including the Centers for Disease Control and Prevention (CDC) and South Carolina Department of Health and Environmental Control (DHEC) to ensure we are apprised of the latest information. Cleaning Products and Protocols: The resort uses cleaning products and protocols which are effective against viruses, including: • Guest Rooms: The Sanctuary and Resort Villas and Homes use cleaning and disinfecting protocols to clean rooms after guests depart and before the next guest arrives, with particular attention paid to high-touch areas and items. -

Preventative Maintenance & Cleaning Guide
Q4 2019 Preventative Maintenance & Cleaning Guide QUICK TIPS & BEST PRACTICES A pristine, prepared property — APPROVED from the bell stand to the back door SUPPLIER While you focus on pleasing your guests, we’ll focus on helping you enhance your image — from retail-inspired uniforms that solidify that important first impression, to well-stocked restrooms complete with sleek designer accessories, to a sparkling lobby • UNIFORM RENTAL that welcomes guests and features fresh custom-logo mats. We’ll also help keep you • FACILITY SERVICES prepared by replenishing your first aid and safety products and much more. • FIRST AID & SAFETY 10% OFF DEEP CLEAN SERVICES performed by Jan 1st, 2020. Ready™ to put your program into place? Contact the Cintas National Service Team at 800.795.7368 or [email protected]. All products and services are provided by Cintas Corporation and not Wyndham Hotels & Resorts (WH&R) or its affiliates. Neither WH&R nor its affiliates are responsible for the accuracy or completeness of any statements made in this advertisement, the content of this advertisement (including the text, representations and illustrations) or any material on the listed supplier’s website to which the advertisement provides a link or a reference. Please refer to the applicable brand standards for your property prior to purchasing products. What’s inside INDEX INTRODUCTION Create a Clean, More Inviting Guest Room Environment ............................... 5 Identifying Common Areas for Guest Complaints ........................................ 6-7 The Hotel Maintenance Doctor is In .................................................................. 8-10 Tips and Tricks For Bathroom Maintenance ........................................................11 The Floor Factor ...................................................................................................... 12-13 The Nose Knows: How to Resolve, Not Mask, Room Odor ......................... -

Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 William A. Rutala, Ph.D., M.P.H.1,2, David J. Weber, M.D., M.P.H.1,2, and the Healthcare Infection Control Practices Advisory Committee (HICPAC)3 1Hospital Epidemiology University of North Carolina Health Care System Chapel Hill, NC 27514 2Division of Infectious Diseases University of North Carolina School of Medicine Chapel Hill, NC 27599-7030 1 Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 3HICPAC Members Robert A. Weinstein, MD (Chair) Cook County Hospital Chicago, IL Jane D. Siegel, MD (Co-Chair) University of Texas Southwestern Medical Center Dallas, TX Michele L. Pearson, MD (Executive Secretary) Centers for Disease Control and Prevention Atlanta, GA Raymond Y.W. Chinn, MD Sharp Memorial Hospital San Diego, CA Alfred DeMaria, Jr, MD Massachusetts Department of Public Health Jamaica Plain, MA James T. Lee, MD, PhD University of Minnesota Minneapolis, MN William A. Rutala, PhD, MPH University of North Carolina Health Care System Chapel Hill, NC William E. Scheckler, MD University of Wisconsin Madison, WI Beth H. Stover, RN Kosair Children’s Hospital Louisville, KY Marjorie A. Underwood, RN, BSN CIC Mt. Diablo Medical Center Concord, CA This guideline discusses use of products by healthcare personnel in healthcare settings such as hospitals, ambulatory care and home care; the recommendations are not intended for consumer use of the products discussed. 2 -

Clean to Dirty
1 High to Low Outside Clean 3 2 to Inside to Dirty Remember the Cleaning Basics There are three basic rules when cleaning a room or an area. 1 Work from the highest point in the room to the lowest point in the room. 2 Work from the outside walls of the room to the center of the room. Work from the cleanest surfaces in the room to the dirtiest surfaces in the 3 room. Table of Contents 1. Bed/Stretcher/Exam Table 2. Blood and Body Fluid Spills 3. Cleaning/Disinfection Solution Mixing 4. Commode/Bedpan/Urinal 5. Damp Mopping 6. Damp Wiping 7. Dry Mopping 8. Exam/ Patient Room: Routine and Discharge 9. Exam/ Patient Room with Precautions: Routine and Discharge 10. Floor Equipment: Use, Care and Maintenance 11. Garbage and Biomedical Waste 12. Kitchen (Staff) 13. Laundry 14. Office 15. Sharps 16. Toys 17. Tub/Shower 18. Waiting Room 19. Washroom 20. Wheelchair 21. Xray Room 1 High Bed/Stretcher/Exam Table to Low Outside Clean 3 2 to Inside to Dirty Remember the Cleaning Basics PURPOSE: To provide a clean stretcher, bed, and exam table for patients MATERIALS: • Disposable gloves • If needed: - Disposable gown - Disposable mask • Prepared Cleaner/Disinfectant solution in cleaning bucket • Container for dirty cloths (if using reusable cleaning cloths) • Cleaning cloths 1 - 1 CLEANING STEPS 1 Do a Risk Assessment • Determine risk of exposure to germs and the Personal Protective Equipment (PPE) required for _the task. • Wear the correct PPE to safely do the job. 2 Cleaning Frequencies • Beds: Clean weekly; if visibly dirty and between each client. -

Classification of Cleaning Agents Cleaning Agents Are Classified According to the Principle Method by Which Soil Or Stains Are Removed from the Surface
Classification of Cleaning Agents Cleaning agents are classified according to the principle method by which soil or stains are removed from the surface. This will be determined by their composition. The principle classes are: • Water • Detergents • Abrasives • Degreasers • Acid cleaners • Organic solvents • Other cleaning agents 1. WATER: Water is the simplest cleaning agent and some form of dirt will be dissolved by it, but normally it is a poor cleaning agent if used alone. It becomes effective only if used in conjunction with some other agent, e.g. a detergent. Water serves to: • Carry the cleaning materials to the soil • Suspend the soil • Remove the suspended soil from the cleaning site • Rinse the detergent solution from the surface • Water has poor power of detergency because: • It has high surface tension and forms droplets • It has little wetting power • It is repelled by oil and grease • If shaken within oil the emulsion does not prevent the formation of large droplets • It has low surfactant effect (surface active agent) 2. DETERGENT: Detergents are those cleaning agents, which contain significant quantities of a group of chemicals known as ‘Surfactants’ (chemicals that have water and soil attracting properties). A number of other chemicals are frequently included to produce detergents suitable for a specific use. A good detergent should – • Reduce the surface tension of water so that the cleaning solution can penetrate the soil • Emulsify soil and lift it from the surface • Be soluble in cold water • Be effective in hard water and a wide range of temperatures. • Be hard on the surface that has to be cleaned. -

Disinfection 101
Disinfection 101 Last Modified: May 2008 Author: Glenda Dvorak, DVM, MS, MPH Portions Reviewed By: James Roth, DVM, PhD, DACVM; Sandra Amass, DVM, PhD, DABVP Center for Food Security and Public Health 2160 Veterinary Medicine Ames, IA 50011 515-294-7189 www.cfsph.iastate.edu Disinfection 101 TABLE OF CONTENTS Introduction......................................................................................................................... 3 Disinfectants Defined............................................................................................................ 3 Disinfectant Labels ............................................................................................................... 4 Label Claims ................................................................................................................................ 4 Other Important Information on a Product Label............................................................................. 5 Considerations and assessment for a disinfection action plan .................................................. 5 Microorganism considerations........................................................................................................ 5 Disinfectant considerations............................................................................................................ 6 Environmental considerations ........................................................................................................ 7 Physical Disinfection .................................................................................................................... -

Laundry Room Cleaning Checklist: Routine Cleaning
LAUNDRY ROOM CLEANING CHECKLIST: ROUTINE CLEANING NAME: ROOM: TIME: DATE: CROSS CONTAMINATION LAUNDERY ROOM CLEANING PREVENTATIVE MEASURES PREPARATION AND SETUP PROCEDURES New mop heads are also used Prepare equipment and load Cleaning when mopping common spaces to Cart with everything needed for the task prevent cross contamination. Survey space, choose direction EPA approved cleaner/detergent to clean (clockwise, EPA approved disinfectant Mop water & mop heads are counterclockwise) straighten Disposable microfiber cloths changed after leaving every space. furnishings and pick up loose Disposable microfiber mop debris Used mop heads and rags are Lint/dust bunnies Disposable microfiber duster placed in a “dirty laundry” bag and Disposable microfiber dust mop Any objects on the floor submitted to laundry service for Extension pole deep cleaning and drying at the end Gloves of the shift. Empty and line waste containers Anti-microbial soap Handle bag from top Hand sanitizer Soiled Gloves removed and placed in trash Clean waste basket Hands cleaned with soap and water Put on the appropriate attire and Perform high dusting with or hand sanitizer. Personal Protective Equipment Microfiber Flexible Dusting Wand (PPE) Eye protection cleaned and Vents (supply & return) Eye protection Facility approved sanitized Light fixtures goggles Sprinkler heads Disposable gloves Face mask assessed for safe High ledges functionality BASIC PROCEDURES Perform hand hygiene and put on gloves before entering the room Leave cleaning -

Environmental Services Cleaning Guidebook
Environmental Services Cleaning Guidebook Adapted from Allina Hospitals and Clinics Environmental Services Cleaning Guidebook by the Minnesota Hospital Association (MHA), Minnesota Department of Health (MDH) and Stratis Health, with representatives from: CentraCare Health – Melrose, Grand Itasca Clinic and Hospital, Minnesota Valley Health Center, Park Nicollet Methodist Hospital, United Hospital, University of Minnesota Medical Center, and Windom Area Hospital, as a part of the “Controlling CDI” project. 1 2 Introduction Clostridium difficile infection (CDI) is becoming more prevalent as a health care-associated infection, causing diarrhea that can lead to colitis, colon perforation, sepsis, and, according to the Centers for Disease Control and Prevention (CDC), is fatal in approximately 14,000 Americans annually. CDC guidelines have been in place nationally for at least five years, targeting antimicrobial stewardship, early identification and treatment, and the prevention of health care facility transmission. A recent study by Sitzlar, et al. (2013) suggested that effective cleaning coupled with staff supervision is a powerful method in decreasing the potential for CDI transmission in hospitals. The Minnesota Hospital Association (MHA), in conjunction with Stratis Health and the Minnesota Department of Health (MDH), has been working with seven Minnesota hospitals: CentraCare Health – Melrose, Grand Itasca Clinic and Hospital, Minnesota Valley Health Center, Park Nicollet Methodist Hospital, United Hospital - a part of Allina Health, University of Minnesota Medical Center, and Windom Area Hospital, to adapt the Allina Health System environmental cleaning training and supervision model for statewide dissemination. The accompanying environmental services cleaning guidebook and training presentation apply to general infection and control principles, with CDI specific recommendations included, such as bleach or other sporicidal disinfectants. -

Guidebook of Part Cleaning Alternatives “Making Cleaning Greener in Massachusetts”
Guidebook of Part Cleaning Alternatives “Making Cleaning Greener in Massachusetts” Principal Authors: Karen Thomas Toxics Use Reduction Institute University of Massachusetts Lowell John Laplante Alan Buckley Office of Technical Assistance for Toxics Use Reduction Executive Office of Environmental Affairs March 1997 Contributors: Carole LeBlanc Jay Jankaskaus Toxics Use Reduction Institute Heather Tenney Sean McGuigan Nathaniel Lloyd Ariadne Kuczwaj Office of Technical Assistance All rights to this report belong to the Toxics Use Reduction Institute and the Office of Technical Assistance for Toxics Use Reduction. The material may be reproduced if attributed to the source. © Toxics Use Reduction Institute, University of Massachusetts Lowell Office of Technical Assistance, Executive Office of Environmental Affairs, Commonwealth of Massachusetts 1997. Acknowledgements This Guidebook is the collaborative effort of many individuals at the Massachusetts Office of Technical Assistance and the Massachusetts Toxics Use Reduction Institute. The original idea for this Guidebook came from meetings of the “Cleaning is Greener” team which was comprised of members from both organizations. The authors would like to thank the members of this team. In addition, the authors would especially like to thank the following individuals for valuable review of and comment on drafts of this work: Barbara Kelley and Rick Reibstein of the Office of Technical Assistance, Ken Geiser of the Toxics Use Reduction Institute, William McGowan and Ken Soltys of the Office of Technical Assistance and Sam Perkins of Green Tech Consulting. ii INTRODUCTION Cleaning dirty parts is an important component of many industrial operations and involves removing of contamination (e.g., dirt, oil, fingerprints, flux) from a surface.