Guidebook of Part Cleaning Alternatives “Making Cleaning Greener in Massachusetts”
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Precision Cleaning Applications
CHAPTER 3 PRECISION CLEANING APPLICATIONS 3.1. BACKGROUND Precision cleaning applications are characterised by the extremely fine level of cleanliness required to keep delicate instruments and surfaces operating effectively. Although precision cleaning is needed in a wide variety of industries, several key factors define the applications where precision cleaning is required. These include those applications in which (1) critical cleanliness standards of particulate and/or organic contaminants need to be satisfied, (2) components have sensitive compatibilities, (3) components I $ have physical characteristics such as geometry and porosity which make dewatering crucial in the cleaning process, and (4) components being cleaned are costly. A variety of particulate and nonparticulate contaminants are removed in precision cleaning operations. Particulate contamination includes scrap material created during cutting, drilling, grinding, and buffing of precision parts (e.g., silicon wafers and aluminum storage disk substrates). Nonparticulate contamination includes waxes, finger print oils, and other contaminants. As the term suggests precision cleaning involves cleaning components to extremely strict standards of cleanliness. A good indication of the cleaning ability required for precision cleaning processes is provided in Figure 111-1, which shows the clearance on a computer disk drive relative to the size of various contaminants. The factor that made CFC-113 the precision cleaning solvent of choice is its remarkable chemical stability (manifested directly in its compatibility to structural materials), its low toxicity, and zero flammability. This has * 1991 UNEP SOLVENTS. COATINGS, AND ADHESIVES REPORT * -1 31- Figure III-I SIZE COMPARISON OF COMPUTER DISK DRIVE HEAD CLEARANCE WITH VARIOUS CONTAMINANTS ' !! allowed closed, superclean, white-room assembly areas to be operated safely and effectively. -

Cleaning with Supercritical Carbon Dioxide
Cleaning with Supercritical Carbon Dioxide Ken Laintz and Dale Spall Los Alamos NATIONAL LABORATORY Chemical Science and Technology Organic Analytical Chemistry CST-12, MS E537 Los Alamos, New Mexico 87545 (505) 665-3545, FAX (505) 667-6561 Outline Supercritical Fluids Cleaning Operations using Supercritial Fluid Solvents Experimental Cleaning Results Other Cleaning Applications Significant Properties of Supercritical Fluids 0 High diffusivity results in high mass transfer 0 Low viscosity and surface tension results in small pore penetration 0 High density results in solvent properties similar to liquids 0 Density is a selective function of temperature and pressure Solute Extraction and Removal Kinetics Extraction Time Operational Economics of Cleaning Prospects Direct Labor Indirect Labor Factory Costs Labor Expenses Benefits Factory Overhead Depreciation Cycle Time Costs Maintenance New Technology Changeover Costs Facility Space Costs Consumables Consumables Disposal Costs Liability Costs Utilities Energy and Utilities Aqueous Supercritical CO2 Taxes and Fees Administration Semi-aqueous Finance Capital Costs Finance Charges Quality Cost of Quality JAAST Joint Association for the Advancement of Supercritical Fluid Technology Mission: To develop and disseminate SCF cleaning applications in support of environmentally conscious manufacturing to meet the needs of the government and industry Members Hughes Aerospace Applied Separations DOE U. Mass. Lowell Allied Signal Autoclave Engineers EPA U. South Carolina Ciba Vision CF Technologies SNL -

Theory of Operation: How Parts Cleaning Works
Theory of Operation: How Parts Cleaning Works MART defines the term to clean as to overpower the soils. The MART Parts Washer is a high-impact pressure, high-temperature, industrial water-based cleaning system that uses a combination of the following factors to achieve cleaning results: Power x Temperature x Chemical x Time = Clean The relationship of these variables can be varied in an infinite number of ways to achieve the same level of cleanliness. Your own needs determine the relative value of each variable. Keep in mind that the MART Parts Washer provides one of the highest blasting powers in the cleaning industry, allowing you to reduce the wash-cycle times for your parts to a minimum. Additionally, the high blasting power allows you to operate the washer at lower cleaning temperatures, thus saving energy, and using less chemical than spray washers The exact combination of the factors must be determined for your application, based on the types of soils to be removed, the degree of cleanliness required, the cycle time required, the types of parts to be cleaned, and so on. How the Parts Washer Works The parts washer operates on a timed cycle. The operator places the parts to be cleaned in the washer on the turntable, closes and latches the door, and then starts the timed cleaning cycle. During the cleaning cycle, a high-temperature, high-pressure, water-and-detergent cleaning solution blasts soils from the parts. After the cycle has stopped and the steam has exhausted, the operator removes the cleaned parts. The parts washer utilizes closed loop, waste minimization technology, continuously reusing its cleaning solution and effectively reducing pollution potential. -

Development and Design of a High Pressure Carbon Dioxide System for the Separation of Hazardous Contaminants from Non-Hazardous Debris*
DEVELOPMENT AND DESIGN OF A HIGH PRESSURE CARBON DIOXIDE SYSTEM FOR THE SEPARATION OF HAZARDOUS CONTAMINANTS FROM NON-HAZARDOUS DEBRIS* Carol L. J. Adkins and Edward M. Russick <~~ 'f\ «//) - cj^'- - / ^ 61 Sandia National Laboratories, Albuquerque, NM -^ " H. Mike Smith and Ron B. Olson AlliedSignal Inc., Kansas City Division, Kansas City, MO ABSTRACT Under the Department of Energy (DOE)AJnited States Air Force (USAF) Memorandum of Understanding, a system is being designed that will use high pressure carbon dioxide for the separation of oils, greases, and solvents from non-hazardous solid waste. The contaminants are dissolved into the high pressure carbon dioxide and precipitated out upon depressurization. The carbon dioxide solvent can then be recycled for continued use. Excellent extraction capability for common manufacturing oils, greases, and solvents has been measured. It has been observed that extraction performance follows the dilution model if a constant flow system is used. The solvents tested are extremely soluble and have been extracted to 100% under both liquid and mild supercritical carbon dioxide conditions. These data are being used to design a 200 liter extraction system. KEY WORDS: Supercritical, Carbon dioxide, Cleaning 1. INTRODUCTION Large quantities of solid wastes such as rags, kimwipes, swabs, coveralls, gloves, etc., contaminated with oils, greases and hazardous solvents are generated by industry and the government. At present, these materials must be treated as hazardous waste, and the cost of disposal of such large volumes of hazardous materials is high. If the hazardous components (oils, greases and solvents) could be segregated from the much larger bulk of non-hazardous material, then these solid materials could potentially be handled as sanitary waste at significant cost savings. -

The Biomatic® Parts Cleaning System Is…
BIOMATIC® The Environmentally Friendly Bioremediation Parts Washer 1 The Biomatic® Parts Cleaning System Is… • A Bioremediation System. The Graymills Biomatic Parts Cleaning System uses the natural technology of microbial bioremediation to reduce oils, greases and other hydrocarbons introduced into the cleaning system to water and carbon dioxide (CO2). No oil and hydrocarbon buildup means the unit continues to clean parts indefinitely (we recommend cleaning the tank once a year, but the fluid can be put back in for continued use if desired.) • A Waste Minimization System. Talking to your customers about “waste minimization” will get their attention. With the Graymills Biomatic Parts Cleaning System, users will go from monthly dumping of fluid and unit cleanup…to once a year. That’s a “waste minimization” sales story few customers can resist. • A Tough Cleaner. Aqueous based Super Biotene® is pH neutral with a mild, pleasant odor. A mixture of emulsifiers and surfactants, they contain no VOCs, so they’re EPA and air quality friendly! Super Biotene cleans grease and grime off even the dirtiest parts, then the microbes go to work, reducing the hydrocarbon contaminants (oils, greases) to water and CO2. • A Good Investment. Recycling costs are slashed…parts cleaner waste is minimized…the dirtiest parts come clean…and the system is operator friendly. 2 How The Biomatic® Parts Cleaning System Works It is important to understand that the Biomatic® Parts Cleaning System is more than individual products - it is a total cleaning environment. The Biomatic Parts Cleaning System relies on the perfect balance between the Super Biotene® cleaning solution and the microbes introduced into the system. -
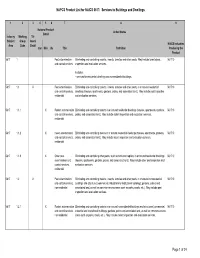
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings 1 2 3 456 7 8 9 National Product United States Detail Industry Working Tri- Subject Group lateral NAICS Industries Area Code Detail Can Méx US Title Definition Producing the Product 5617 1 Pest extermination Eliminating and controlling rodents, insects, termites and other pests. May include termite/pest 561710 and control services inspection and evaluation services. Includes: • services for residential dwellings and nonresidential buildings. 5617 1.1 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around residential 561710 and control services, dwellings (houses, apartments, gardens, patios, and associated land). May include pest inspection residential and evaluation services. 5617 1.1.1 X Rodent extermination Eliminating and controlling rodents in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include rodent inspection and evaluation services. residential 5617 1.1.2 X Insect extermination Eliminating and controlling insects in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include insect inspection and evaluation services. residential 5617 1.1.9 X Other pest Eliminating and controlling other pests. such as birds and reptiles, in or around residential dwellings 561710 extermination and (houses, apartments, gardens, patios, and associated land). May include other pest inspection and control services, evaluation services. residential 5617 1.2 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around nonresidential 561710 and control services, buildings and structures (commercial, industrial and institutional buildings; gardens, patios and nonresidential associated land, as well as common-access areas such as parks, roads, etc.). -

We Take Standards for Hygiene and Cleanliness Very Seriously and Are Taking Additional Steps to Ensure the Safety of Our Guests and Associates
We take standards for hygiene and cleanliness very seriously and are taking additional steps to ensure the safety of our guests and associates. On a daily basis, associates at our hotel, villas and homes, clubhouses, nature center and other public areas are working to ensure that they meet the latest guidance on hygiene and cleaning. Our health and safety measures are designed to address a broad spectrum of viruses, including COVID-19, and include everything from frequent handwashing hygiene and cleaning product specifications to guest room and common area cleaning procedures. Specific steps Kiawah Island Golf Resort is taking include: Associate Health, Safety and Knowledge: Resort associates – and their own health, safety and knowledge – are essential to an effective cleaning program. Here are some ways we’re supporting them: • Hand Hygiene: Proper and frequent handwashing is vital to help combat the spread of viruses. In our daily meetings, our teams are reminded that cleanliness starts with this simple act. It’s important for their health and that of our guests. • Ongoing Training: In addition to training on housekeeping and hygiene protocols, resort associates are also completing enhanced COVID-19 awareness training. • Real Time Information: Kiawah Island Golf Resort continues to coordinate with national, regional, state and local authorities, including the Centers for Disease Control and Prevention (CDC) and South Carolina Department of Health and Environmental Control (DHEC) to ensure we are apprised of the latest information. Cleaning Products and Protocols: The resort uses cleaning products and protocols which are effective against viruses, including: • Guest Rooms: The Sanctuary and Resort Villas and Homes use cleaning and disinfecting protocols to clean rooms after guests depart and before the next guest arrives, with particular attention paid to high-touch areas and items. -

Line Card Listing by Vendor
KAMPI COMPONENTS - Line Card by Manufacturer / OEM 20-Mar-18 Cage Code Manufacturer / OEM Product Line(s) 08060 A & J Manufacturing Co. Equipment racks and enclosures 6Z800 A & M Aerospace Inc. Aerospace components manufacture 00144 A.D.C. Products Division Electrical cords and related products 46FH0 A-1 Fire Equipment Fire suppression systems 15755 Abbott Transistors Power supplies and inverters 3M927 Accurate Rubber Products, Inc. Grommets, bumpers, suction cups, o-rings, seals 51506 Accurate Screw Machine Company Electronic hardware & fasteners 0TYP8 Accushim Inc. Stainless steel shims and shim kits 0LLR0 Accu-Wire & Cable Wire and cable products 83908 Ace Products Company Windshield wipers, scrapper rings 0HCJ7 Ace Rubber Products Molded rubber products 47181 Aces Systems-Dynamic Instruments Vibration analysis instrumentation and products 18560 ACR Electronics Inc. Electronic VHF/UHF radio sets, strobe light beacons 57598 ACS Industries, Inc. Engineered knitted wire mesh products 66173 Addco Mfg. Co. Large mobile and fixed LED displays 03RL1 Advanced Cable Ties, Inc. Cable ties and wiring products 60642 Advanced Conversion Technology Military power supplies 1ED54 Advanced Seal Technology Mechanical seals; labyrinth, split design, pressure, vacuum and temperature 09332 Aerofast Inc. Quick release pins and attachments 82366 Aero-Motive Mfg. Co. Electric cord and cable reels, hose reels, winding reels 98810 Aerosonic Corp. Aeronautical flight instruments, watches, clocks and aircraft instruments 0E0Z8 Aerospace Mfg. Inc. Fasteners for aerospace and military applications 17140 Aerospace Products, Inc. Electronic and jet engine parts, aerospace and aircraft fasteners 4F124 Aircraft Fasteners Inc. Self-locking nuts, inserts and related fastener products for aerospace applications 0CD56 Aircraft Hinge Inc. Aviation hinges and hinge assemblies 32970 Aircraft Parts Co. -

Traditional Aqueous Parts-Cleaning Operations Work Great on Day One, but Rapidly Become Contaminated
➤ B Y S tep H en D . te M ple , ran S O H O FF Cleaning you can count on Traditional aqueous parts-cleaning operations work great on day one, but rapidly become contaminated. Adding an ultrafiltration process to a parts cleaning system can dramatically extend and improve its performance. Traditional aqueous parts-cleaning operations can be inconsistent and costly. Machine shops and contract manufacturers dealing with a range of contaminants and heavy contaminant loads are finding they need something more. In many cases, adding an ultrafil- tration (UF) system to an existing parts cleaning system can provide cleaner parts as well as dramatically lower maintenance and operating costs. This is not to say that traditional sys- tems cannot be effective in certain situ- ations. For example, a shop can realize good bath life and part cleanliness when primarily cleaning with non- emulsified oils and when the system’s cleaning chemistry can effectively split the oil to the surface of the parts bath. This splitting allows removal via con- All images: Ransohoff ventional oil skimming or decanting Deerfield Manufacturing installed a 360 gal./day UF system that effectively turns methods. (A typical industrial parts over its wash tank every fourth day. washer includes a wash stage where most contaminants are removed, fol- rity and achieve optimal cleaning. The This is an inefficient and inconsistent lowed by rinsing and drying stages. result is that metalworking operations process, particularly for operations that Newer systems may include only a start out on day one with fresh water must meet stringent parts cleanliness single-stage wash with dry off.) and a new chemical charge to create specifications, such as in Six Sigma However, a more likely scenario for the cleaning solution. -

Aero-Green Parts Washer Degreaser -4160-4150
Aero-Green Parts Washer Degreaser -4160-4150 Aero-Green 4150-4160 Parts Washer Concentrated Degreaser for use in Immersion Degreasers: Ultrasonic Degreaser, Spray Cabinet Washer, Adjulift Parts Washer, Glove Box Washers and Hot & Cold Standard parts washers. Cleans Virtually All Metals Including Steels, Aluminum & Titanium SPRAY CABINET WASHERS Spray Cabinet Washers are designed to clean large parts that are placed directly on a retractable turntable or batches of small parts loaded in baskets. This heavy-duty industrial parts washer combines pressure, heat and aqueous cleaning solution such as Aero-Green 4150-4160 to clean and degrease parts to spec within minutes. Many machines are designed with added features such as low-maintenance pumps, digital temperature controllers, and cycle timers, low water shut off, automatic fill, proportioners, oils skimmers and fresh water rinses. Each recirculate stage has its dedicated tank, heating system, circulation pump, cycle controls and spray manifolds in the main spray cabinet. Typhoon® HD is an ideal aqueous parts washer for in-process and final cleaning in maintenance, manufacturing and remanufacturing applications. Spray Cabinet Washers deliver deep-down auto parts cleaning designed for durability and precision under the most demanding conditions. The powerful force of the jets and the Aero-Green 4150- 4160 combine to remove grease, oil, carbon and other grime in just minutes. Spray Cabinet Washer technology, is effectively used in industries ranging from remanufactured railroad drivetrain parts to in-process cleaning for jet engine components Spray Cabinet Washers are designed to clean, degrease bulk loads of small or large parts in preparation for surface treatment, packaging and distribution. -

Clean Ozzyjuice Means Clean Parts
® OZZYMAT The SmartWasher® Bioremediating Parts Washing System is both self-cleaning and non-hazardous. Through the process of bioremediation the SMARTWASHER constantly maintains the cleanliness of the OZZYJUICE cleaning/degreasing solution without the need for hauling, skimming or evacuating the unit. WHAT IS OZZYJUICE®? OZZY ® OZZYJUICE is a powerful, aqueous based degreasing MICROBES Ozzy Ozzy ® Microbe solution that is pH-neutral, non-irritating, non-flammable, and non-toxic. Armed with superior cleaning capability, the OZZYJUICE solutions will match or surpass the performance of a premium solvent without all the associated risks. All HEATED OZZYJUICE OZZYJUICE solutions are certified by both NSF and AQMD. HEATING 300 GPH ELEMENT RECIRCULATING OZZY® MICROBES KEEP THE OZZYJUICE CLEAN AND STRONG. PUMP MODELS Microbes (OZZYS), introduced into the SMARTWASHER 14161 14145 14711 system through the OZZYMAT, eat the grease, oil and other contaminants, cleaning off the dirty parts, creating the harmless by-products of carbon dioxide and water. CLEAN OZZYJUICE MEANS CLEAN PARTS. SW-23 SW-25 SW-25C Mobile Parts/Brake Signature Parts Combo Brake/Parts Washer Washer Washer The OZZYJUICE cleaning/degreasing solution cleans the dirty 14144 14710 14162 parts and the OZZY microbes clean the OZZYJUICE leaving it strong and clean for every use. You should never need to haul away dirty parts cleaning fluid again, if the unit is maintained properly, and according to the operating instructions. Ozzy® SW-28 SW-28XE SW-37 ® Microbe Ozzy SuperSink Parts SuperSink XE Parts Mobile Heavyweight Washer Washer Parts Washer www.crcindustries.com/ei | 885 Louis Drive, Warminster, PA 18974, (PH) 800-272-4620 Copyright © 2015 ChemFree Corporation, a subsidiary of CRC Industries. -

Supercritical Fluid Carbon Dioxide Cleaning of Plutonium Parts
I SUPERCRITICAL FLUID CARBON DIOXIDE BACKGROUND CLEANING OF PLUTONIUM PARTS A supercritical fluid is the comprcssed, dense gas phase Stephanie J. Hale above the critical temperature. Liquefaction of a gas can EG&G Rocky Flats, Inc occur upon compression below the critical temperature, but above the critical temperature the gas cannot liquefy regardleas of the applied pressure and a single gas phase is ABSTRACT maintained. The critical point for carbon dioxide is 31'C and 74 bar (1088 psi) which means that the supercritical Supercritical fluid carbon dioxide is under investigation in fluid can be aUnined under relatively mild conditions. this work for use as a cleaning solvent for the final cleaning of plutonium parts. These parts must be free of The phase diagram of CO, (Figure 1) shows the organic residue to avoid cormsion in the stockpile. Initial supcrcritical region above 74 bar and the 32" isotherm. It studies on stainless steel and full-scale mock-up parts can be seen that very liquid-like densities can be achieved indicate that the oils of interest are easily and adequately and still remain in the gas phase. cleaned from the metal surfaces with supercritical fluid carbon dioxide. Results from compatibility studies show that undesirable oxidation or other surface mctions are not occurring during cxposureof plutonium to the supercritical fluid. Cleaning studies indicate that the oils of interest are Temperalure ' removed from the plutonium surface under relatively mild conditions. These studies indicate that supercritical fluid carbon dioxide is a very promising cleaning medium for this application. INTRODUCTION Our aim in this work is to develop a cleaning process that van u-o clean plutonium parts.