Disinfection 101
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Disinfectants for Biohazardous Materials (3/21)
Safe Operating Procedure (Revised 3/21) CHEMICAL DISINFECTANTS FOR BIOHAZARDOUS MATERIALS ____________________________________________________________________________ Chemicals used for biohazardous decontamination are called sterilizers, disinfectants, sanitizers, antiseptics and germicides. These terms are sometimes equivalent, but not always, but for the purposes of this document all the chemicals described herein are disinfectants. The efficacy of every disinfectant is based on several factors: 1) organic load (the amount of dirt and other contaminants on the surface), 2) microbial load, 3) type of organism, 4) condition of surfaces to be disinfected (i.e., porous or nonporous), and 5) disinfectant concentration, pH, temperature, contact time and environmental humidity. These factors determine if the disinfectant is considered a high, intermediate or low-level disinfectant, in that order. Prior to selecting a specific disinfectant, consider the relative resistance of microorganisms. The following table provides information regarding chemical disinfectant resistance of various biological agents. Microbial Resistance to Chemical Disinfectants: Type of Microbe Examples Resistant Bovine spongiform encephalopathy (Mad Prions Cow) Creutzfeldt-Jakob disease Bacillus subtilis; Clostridium sporogenes, Bacterial Spores Clostridioides difficile Mycobacterium bovis, M. terrae, and other Mycobacteria Nontuberculous mycobacterium Poliovirus; Coxsackievirus; Rhinovirus; Non-enveloped or Small Viruses Adenovirus Trichophyton spp.; Cryptococcus sp.; -

EH&S COVID-19 Chemical Disinfectant Safety Information
COVID-19 CHEMICAL DISINFECTANT SAFETY INFORMATION Updated June 24, 2020 The COVID-19 pandemic has caused an increase in the number of disinfection products used throughout UW departments. This document provides general information about EPA-registered disinfectants, such as potential health hazards and personal protective equipment recommendations, for the commonly used disinfectants at the UW. Chemical Disinfectant Base / Category Products Potential Hazards Controls ● Ethyl alcohol Highly flammable and could form explosive Disposable nitrile gloves Alcohols ● ● vapor/air mixtures. ● Use in well-ventilated areas away from o Clorox 4 in One Disinfecting Spray Ready-to-Use ● May react violently with strong oxidants. ignition sources ● Alcohols may de-fat the skin and cause ● Wear long sleeve shirt and pants ● Isopropyl alcohol dermatitis. ● Closed toe shoes o Isopropyl Alcohol Antiseptic ● Inhalation of concentrated alcohol vapor 75% Topical Solution, MM may cause irritation of the respiratory tract (Ready to Use) and effects on the central nervous system. o Opti-Cide Surface Wipes o Powell PII Disinfectant Wipes o Super Sani Cloth Germicidal Wipe 201 Hall Health Center, Box 354400, Seattle, WA 98195-4400 206.543.7262 ᅵ fax 206.543.3351ᅵ www.ehs.washington.edu ● Formaldehyde Formaldehyde in gas form is extremely Disposable nitrile gloves for Aldehydes ● ● flammable. It forms explosive mixtures with concentrations 10% or less ● Paraformaldehyde air. ● Medium or heavyweight nitrile, neoprene, ● Glutaraldehyde ● It should only be used in well-ventilated natural rubber, or PVC gloves for ● Ortho-phthalaldehyde (OPA) areas. concentrated solutions ● The chemicals are irritating, toxic to humans ● Protective clothing to minimize skin upon contact or inhalation of high contact concentrations. -

Decontamination of Rooms, Medical Equipment and Ambulances Using an Aerosol of Hydrogen Peroxide Disinfectant B.M
Journal of Hospital Infection (2006) 62, 149–155 www.elsevierhealth.com/journals/jhin Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant B.M. Andersena,*, M. Rascha, K. Hochlina, F.-H. Jensenb, P. Wismarc, J.-E. Fredriksend aDepartment of Hospital Infection, Ulleva˚l University Hospital, Oslo, Norway bDivision of Pre-hospital Care, Ulleva˚l University Hospital, Oslo, Norway cDepartment of Medical Equipment, Ulleva˚l University Hospital, Oslo, Norway dHealth and Environment AS, Oslo, Norway Received 17 November 2004; accepted 1 July 2005 KEYWORDS Summary A programmable device (Sterinis, Gloster Sante Europe) Room decontamina- providing a dry fume of 5% hydrogen peroxide (H2O2) disinfectant was tion; Ambulance tested for decontamination of rooms, ambulances and different types of decontamination; medical equipment. Pre-set concentrations were used according to the Medical equipment decontamination; volumes of the rooms and garages. Three cycles were performed with Hydrogen peroxide increasing contact times. Repetitive experiments were performed using fume decontamina- Bacillus atrophaeus (formerly Bacillus subtilis) Raven 1162282 spores to tion; Spore test control the effect of decontamination; after a sampling plan, spore strips were placed in various positions in rooms, ambulances, and inside and outside the items of medical equipment. Decontamination was effective in 87% of 146 spore tests in closed test rooms and in 100% of 48 tests in a surgical department when using three cycles. One or two cycles had no effect. The sporicidal effect on internal parts of the medical equipment was only 62.3% (220 tests). When the devices were run and ventilated during decontamination, 100% (57/57) of spore strips placed inside were decontaminated. -

Use of 90% Ethanol to Decontaminate Stethoscopes in Resource Limited
Raghubanshi et al. Antimicrobial Resistance and Infection Control (2017) 6:68 DOI 10.1186/s13756-017-0224-x RESEARCH Open Access Use of 90% ethanol to decontaminate stethoscopes in resource limited settings Bijendra Raj Raghubanshi, Supriya Sapkota, Arjab Adhikari*, Aman Dutta, Utsuk Bhattarai and Rastriyata Bhandari Abstract Background: In developing countries like Nepal, 90% ethanol is cheap and is available in most hospitals. The unavailability of isopropyl alcohol (IPA) in these settings led us to compare the efficacy between 90% ethanol and isopropyl alcohol pads in reducing the bacterial contamination of diaphragm of stethoscope. Methods: A randomized blinded experimental study was carried out to determine the difference between cleaning stethoscopes with 90% ethanol and IPA. Cultures of diaphragm were taken before and after cleaning with one of the cleaning agent. Colony forming units (CFU) count and organism identification was done by a blinded investigator. CFU before and after cleaning were compared using Wilcoxon signed–rank test. Mann Whitney U test was used to compare the decrease in CFU count between the cleaning agents. Results: About 30% of the stethoscopes harbored potential pathogens. Significant reduction in CFU was observed with both IPA (Wilcoxon signed–rank test, P value <0.001) and 90% ethanol (Wilcoxon signed–rank test, P value <0. 001). Comparing median decrease in CFU between cleaning with IPA and with 90% ethanol, no significant difference was found (Mann Whitney U test; U = 1357, P value >0.05). Conclusions: Both 90% ethanol and IPA are equally effective in decontaminating the diaphragm of stethoscope. Selection of agent should be done on the basis of cost and availability. -

Theory of Operation: How Parts Cleaning Works
Theory of Operation: How Parts Cleaning Works MART defines the term to clean as to overpower the soils. The MART Parts Washer is a high-impact pressure, high-temperature, industrial water-based cleaning system that uses a combination of the following factors to achieve cleaning results: Power x Temperature x Chemical x Time = Clean The relationship of these variables can be varied in an infinite number of ways to achieve the same level of cleanliness. Your own needs determine the relative value of each variable. Keep in mind that the MART Parts Washer provides one of the highest blasting powers in the cleaning industry, allowing you to reduce the wash-cycle times for your parts to a minimum. Additionally, the high blasting power allows you to operate the washer at lower cleaning temperatures, thus saving energy, and using less chemical than spray washers The exact combination of the factors must be determined for your application, based on the types of soils to be removed, the degree of cleanliness required, the cycle time required, the types of parts to be cleaned, and so on. How the Parts Washer Works The parts washer operates on a timed cycle. The operator places the parts to be cleaned in the washer on the turntable, closes and latches the door, and then starts the timed cleaning cycle. During the cleaning cycle, a high-temperature, high-pressure, water-and-detergent cleaning solution blasts soils from the parts. After the cycle has stopped and the steam has exhausted, the operator removes the cleaned parts. The parts washer utilizes closed loop, waste minimization technology, continuously reusing its cleaning solution and effectively reducing pollution potential. -

Evaluating Disinfectants for Use Against the COVID-19 Virus
When it comes to choosing a disinfectant to combat the COVID-19 virus, research and health authorities suggest not all disinfectants are equally effective. The difference is in their active ingredient(s). HEALTH CANADA AND U.S. EPA ASSESSMENTS The work to evaluate disinfectants perhaps best starts with lists of approved disinfectants compiled by government health authorities. Health Canada has compiled a list of 85 hard surface disinfectant products (as of March 20, 2020) that meet their requirements for disinfection of emerging pathogens, including the virus that causes COVID-19. It can be accessed here. You can wade through the entire list. But if you locate the Drug Identification Number (DIN) on the disinfectant product label or the safety data sheet (SDS), then you can use the search function to quickly see if the product meets Health Canada requirements. A second list, updated on March 19, 2020, provides 287 products that meet the U.S. Environmental Protection Agency’s (EPA) criteria for use against SARS-CoV-2, the novel coronavirus that causes the disease COVID-19. This list can be found here. Like the Health Canada list, you can wade through this one too. However, to best use this list, you should locate the U.S. EPA registration number on the product label or SDS, and use that number to search the list. The U.S. EPA registration number of a product consists of two sets of numbers separated by a hyphen. The first set of numbers refers to the company identification number, and the second set of numbers following the hyphen represents the product number. -

Chemical Disinfectant and Cleaning Agent Safety – Frequently Asked Questions
Caribbean Public Health Agency Technical Guidance: COVID-19 Series No 33 Chemical Disinfectant and Cleaning Agent Safety – Frequently Asked Questions Suggested Citation: CARPHA. (2020). Chemical disinfectant and cleaning agent safety – frequently asked questions. Caribbean Public Health Agency Technical Guidance: COVID-19 Series, no. 33. CARPHA 2020 Chemical Disinfectant and Cleaning Agent Safety – Frequently Asked Questions 9 July 2020 Why are we concerned about cleaning agents and disinfectants? Selecting the correct chemical agent for cleaning and disinfecting and safely handling and using that agent is essential to health and wellbeing. Recently, with the increased focus on cleaning to reduce the risk of exposure to coronavirus disease, there have been an associated increase in media stories warning the public of the dangerous chemicals used for disinfectants. All chemical cleaning agents can be harmful to the health of the person using the product if it is not used as directed on the label by the manufacturer. Chemical agents can cause burns, breathing difficulties or damage to the lungs, or possibly result in poisoning. Persons should carefully read instructions for use, safety data sheets, and any health warnings to ensure they have selected the correct chemical agents, have the appropriate personal protection, and use and store the chemicals in a safe way. What are the properties of effective chemical cleaning agents and disinfectants? A wide variety of chemical agents can be used for cleaning, sanitising, and disinfecting. There are 4 categories of cleaners: detergents, degreasers, abrasives, and acids. Each is effective for cleaning different surfaces depending on the type of soiling. Different chemical agents are effective for killing different bacteria and viruses.1 Molds and fungus will require a sporicidal agent2. -

Comparison of Effectiveness Disinfection of 2%
ORIGINAL RESEARCH Journal of Dentomaxillofacial Science (J Dentomaxillofac Sci ) December 2018, Volume 3, Number 3: 169-171 P-ISSN.2503-0817, E-ISSN.2503-0825 Comparison of effectiveness disinfection of 2% Original Research glutaraldehyde and 4.8% chloroxylenol on tooth extraction instruments in the Department of Oral CrossMark http://dx.doi.org/10.15562/jdmfs.v3i2.794 Maxillofacial Surgery, Faculty of Dentistry, University of North Sumatera Month: December Ahyar Riza,* Isnandar, Indra B. Siregar, Bernard Volume No.: 3 Abstract Objective: To compare disinfecting effectiveness of 2% glutaraldehyde while the control group was treated with 4.8% chloroxylenol. Each Issue: 2 and 4.8% chloroxylenol on tooth extraction instruments at the instrument was pre-cleaned using a brush, water and soap for both Department of Oral Surgery, Faculty of Dentistry, University of North groups underwent the disinfection process. Sumatera. Results: The results were statistically analyzed using Mann-Whitney Material and Methods: This was an experimental study with post- Test. The comparison between glutaraldehyde and chloroxylenol First page No.: 147 test only control group design approach. Purposive technique is showed a significant difference to the total bacteria count on applied to collect samples which are lower molar extraction forceps. In instrument after disinfection (p=0.014 < 0.05). this study, sample were divided into 2 groups and each consisting of 18 Conclusion: 2% glutaraldehyde was more effective than 4.8% P-ISSN.2503-0817 instruments. The treatment group was treated with 2% glutaraldehyde chloroxylenol at disinfecting lower molar extraction forceps. Keyword: Disinfection, Glutaraldehyde, Chloroxylenol, Forceps E-ISSN.2503-0825 Cite this Article: Riza A, Siregar IB, Isnandar, Bernard. -

The Biomatic® Parts Cleaning System Is…
BIOMATIC® The Environmentally Friendly Bioremediation Parts Washer 1 The Biomatic® Parts Cleaning System Is… • A Bioremediation System. The Graymills Biomatic Parts Cleaning System uses the natural technology of microbial bioremediation to reduce oils, greases and other hydrocarbons introduced into the cleaning system to water and carbon dioxide (CO2). No oil and hydrocarbon buildup means the unit continues to clean parts indefinitely (we recommend cleaning the tank once a year, but the fluid can be put back in for continued use if desired.) • A Waste Minimization System. Talking to your customers about “waste minimization” will get their attention. With the Graymills Biomatic Parts Cleaning System, users will go from monthly dumping of fluid and unit cleanup…to once a year. That’s a “waste minimization” sales story few customers can resist. • A Tough Cleaner. Aqueous based Super Biotene® is pH neutral with a mild, pleasant odor. A mixture of emulsifiers and surfactants, they contain no VOCs, so they’re EPA and air quality friendly! Super Biotene cleans grease and grime off even the dirtiest parts, then the microbes go to work, reducing the hydrocarbon contaminants (oils, greases) to water and CO2. • A Good Investment. Recycling costs are slashed…parts cleaner waste is minimized…the dirtiest parts come clean…and the system is operator friendly. 2 How The Biomatic® Parts Cleaning System Works It is important to understand that the Biomatic® Parts Cleaning System is more than individual products - it is a total cleaning environment. The Biomatic Parts Cleaning System relies on the perfect balance between the Super Biotene® cleaning solution and the microbes introduced into the system. -
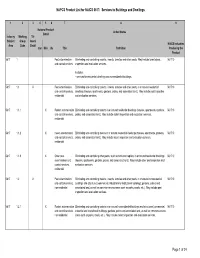
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings 1 2 3 456 7 8 9 National Product United States Detail Industry Working Tri- Subject Group lateral NAICS Industries Area Code Detail Can Méx US Title Definition Producing the Product 5617 1 Pest extermination Eliminating and controlling rodents, insects, termites and other pests. May include termite/pest 561710 and control services inspection and evaluation services. Includes: • services for residential dwellings and nonresidential buildings. 5617 1.1 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around residential 561710 and control services, dwellings (houses, apartments, gardens, patios, and associated land). May include pest inspection residential and evaluation services. 5617 1.1.1 X Rodent extermination Eliminating and controlling rodents in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include rodent inspection and evaluation services. residential 5617 1.1.2 X Insect extermination Eliminating and controlling insects in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include insect inspection and evaluation services. residential 5617 1.1.9 X Other pest Eliminating and controlling other pests. such as birds and reptiles, in or around residential dwellings 561710 extermination and (houses, apartments, gardens, patios, and associated land). May include other pest inspection and control services, evaluation services. residential 5617 1.2 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around nonresidential 561710 and control services, buildings and structures (commercial, industrial and institutional buildings; gardens, patios and nonresidential associated land, as well as common-access areas such as parks, roads, etc.). -

VOLUME 2 Cleaning Agents, Sanitisers and Disinfectants in Food Businesses: Detection of Traces and Human Risk Assessment Processes
Chemicals in Food Hygiene VOLUME 2 Cleaning agents, sanitisers and disinfectants in food businesses: detection of traces and human risk assessment processes Chemicals in Food Hygiene – Volume 2 Foreword The Global Food Safety Initiative (GFSI) is a considering carry-over risks of traces in non-profit industry association tasked with foods; promoting continuous improvement of food ➢ Reviewed and identified gaps in the safety management systems to ensure suitability of existing methods for confidence in the delivery of safe food to detection of traces at relevant points in consumers worldwide. GFSI provides a the food production process; platform for collaboration between some of ➢ Developed the GFSI position on the use of the world’s leading food safety experts from cleaning agents, sanitisers and retailer, producers and food service disinfectants and the relationship with companies, service providers associated with microbial resistance. the food supply chain, international organisations, academia and government. The TWG produced 2 volumes within one document: Since GFSI’s inception in 2000, experts from all over the world have been collaborating in ➢ Volume one of this document provides a numerous Technical Working Groups (TWG) high-level overview of the considerations to tackle current food safety issues defined that a food business operator needs to by GFSI stakeholders. In 2017 a TWG was consider in relation to ensuring established to determine best practices in appropriate hygienic practices. This relation to biocides (defined as the residues volume is aimed at a variety of readers from cleaning agents, sanitisers and from the food truck operator or farmer disinfectants) in the food supply chain. -

European Contact Lens Society of Ophthalmologists ECLSO
European Contact Lens Society of Ophthalmologists ECLSO Course de Base en Contactologie I – IV Basiskurs in Kontaktologie I - IV Dr. med. Albert Franceschetti, Genève Michael Bärtschi, M.Sc.Optom. et M.Med.Educ.,Bern Optics and Contact Lenses What should I know about optics and physiological optics when I fit contact lenses ? Visual acuity = 1.0 • US notation 20/20 (20 feet) • Metric notation 6/6 (6 meters) • Decimal 10/10 (Monoyer) • Logarythmic Distance spectacles-eye • The position of the contact lens is different in relation to the eye. This is obviously the first criterion to take into consideration. • According to the power of the spectacles, the difference ǻ between the power of spectacle lenses and the contact lenses will be more or less important. Formula • DL power of the glasses (supposed perfect DL correction) DCL = -------------- 1 – d D • DCL power of the contact L lens (lens + tear meniscus) • d distance between the lens and the contact lens • ǻ difference between the power DCL and DL ǻ • ǻ = DCL –DL is always positive • ǻ = DCL –DL increases if the distance eye- glasses increases • The value d can be superior to 12mm if one uses trial lenses or refractor. Variation of powers between the two systems (contact lenses and spectacle) Distance eye- DL -20 -15 -10 -8 -5 +4 +10 glasses -16.13 -12.71 -8.93 -7.30 -4.72 +4.20 +11.36 DCL d=12m 3.87 2.29 1.07 0.70 0.28 0.20 1.36 m ǻ -15.38 -12.24 -8.70 -7.14 -4.65 +4.26 +11.76 DCL D=15 ǻ 4.62 2.76 1.30 0.86 0.35 0.26 1.76 mm Magnification • The distance eye-spectacles versus eye contact lens being different, the magnification of the retinal image will be different.