Classification of Cleaning Agents Cleaning Agents Are Classified According to the Principle Method by Which Soil Or Stains Are Removed from the Surface
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sodium Dodecyl Sulfate
Catalog Number: 102918, 190522, 194831, 198957, 811030, 811032, 811033, 811034, 811036 Sodium dodecyl sulfate Structure: Molecular Formula: C12H25NaSO4 Molecular Weight: 288.38 CAS #: 151-21-3 Synonyms: SDS; Lauryl sulfate sodium salt; Dodecyl sulfate sodium salt; Dodecyl sodium sulfate; Sodium lauryl sulfate; Sulfuric acid monododecyl ester sodium salt Physical Appearance: White granular powder Critical Micelle Concentration (CMC): 8.27 mM (Detergents with high CMC values are generally easy to remove by dilution; detergents with low CMC values are advantageous for separations on the basis of molecular weight. As a general rule, detergents should be used at their CMC and at a detergent-to-protein weight ratio of approximately ten. 13,14 Aggregation Number: 62 Solubility: Soluble in water (200 mg/ml - clear, faint yellow solution), and ethanol (0.1g/10 ml) Description: An anionic detergent3 typically used to solubilize8 and denature proteins for electrophoresis.4,5 SDS has also been used in large-scale phenol extraction of RNA to promote the dissociation of protein from nucleic acids when extracting from biological material.12 Most proteins bind SDS in a ratio of 1.4 grams SDS to 1 gram protein. The charges intrinsic to the protein become insignificant compared to the overall negative charge provided by the bound SDS. The charge to mass ratio is essentially the same for each protein and will migrate in the gel based only on protein size. Typical Working Concentration: > 10 mg SDS/mg protein Typical Buffer Compositions: SDS Electrophoresis -

Use of 90% Ethanol to Decontaminate Stethoscopes in Resource Limited
Raghubanshi et al. Antimicrobial Resistance and Infection Control (2017) 6:68 DOI 10.1186/s13756-017-0224-x RESEARCH Open Access Use of 90% ethanol to decontaminate stethoscopes in resource limited settings Bijendra Raj Raghubanshi, Supriya Sapkota, Arjab Adhikari*, Aman Dutta, Utsuk Bhattarai and Rastriyata Bhandari Abstract Background: In developing countries like Nepal, 90% ethanol is cheap and is available in most hospitals. The unavailability of isopropyl alcohol (IPA) in these settings led us to compare the efficacy between 90% ethanol and isopropyl alcohol pads in reducing the bacterial contamination of diaphragm of stethoscope. Methods: A randomized blinded experimental study was carried out to determine the difference between cleaning stethoscopes with 90% ethanol and IPA. Cultures of diaphragm were taken before and after cleaning with one of the cleaning agent. Colony forming units (CFU) count and organism identification was done by a blinded investigator. CFU before and after cleaning were compared using Wilcoxon signed–rank test. Mann Whitney U test was used to compare the decrease in CFU count between the cleaning agents. Results: About 30% of the stethoscopes harbored potential pathogens. Significant reduction in CFU was observed with both IPA (Wilcoxon signed–rank test, P value <0.001) and 90% ethanol (Wilcoxon signed–rank test, P value <0. 001). Comparing median decrease in CFU between cleaning with IPA and with 90% ethanol, no significant difference was found (Mann Whitney U test; U = 1357, P value >0.05). Conclusions: Both 90% ethanol and IPA are equally effective in decontaminating the diaphragm of stethoscope. Selection of agent should be done on the basis of cost and availability. -

Theory of Operation: How Parts Cleaning Works
Theory of Operation: How Parts Cleaning Works MART defines the term to clean as to overpower the soils. The MART Parts Washer is a high-impact pressure, high-temperature, industrial water-based cleaning system that uses a combination of the following factors to achieve cleaning results: Power x Temperature x Chemical x Time = Clean The relationship of these variables can be varied in an infinite number of ways to achieve the same level of cleanliness. Your own needs determine the relative value of each variable. Keep in mind that the MART Parts Washer provides one of the highest blasting powers in the cleaning industry, allowing you to reduce the wash-cycle times for your parts to a minimum. Additionally, the high blasting power allows you to operate the washer at lower cleaning temperatures, thus saving energy, and using less chemical than spray washers The exact combination of the factors must be determined for your application, based on the types of soils to be removed, the degree of cleanliness required, the cycle time required, the types of parts to be cleaned, and so on. How the Parts Washer Works The parts washer operates on a timed cycle. The operator places the parts to be cleaned in the washer on the turntable, closes and latches the door, and then starts the timed cleaning cycle. During the cleaning cycle, a high-temperature, high-pressure, water-and-detergent cleaning solution blasts soils from the parts. After the cycle has stopped and the steam has exhausted, the operator removes the cleaned parts. The parts washer utilizes closed loop, waste minimization technology, continuously reusing its cleaning solution and effectively reducing pollution potential. -

Tutorial on Working with Micelles and Other Model Membranes
Tutorial on Working with Micelles and Model Membranes Chuck Sanders Dept. of Biochemistry, Dept. of Medicine, and Center for Structural Biology Vanderbilt University School of Medicine. http://structbio.vanderbilt.edu/sanders/ March, 2017 There are two general classes of membrane proteins. This presentation is on working with integral MPs, which traditionally could be removed from the membrane only by dissolving the membrane with detergents or organic solvents. Multilamellar Vesicles: onion-like assemblies. Each layer is one bilayer. A thin layer of water separates each bilayer. MLVs are what form when lipid powders are dispersed in water. They form spontaneously. Cryo-EM Micrograph of a Multilamellar Vesicle (K. Mittendorf, C. Sanders, and M. Ohi) Unilamellar Multilamellar Vesicle Vesicle Advances in Anesthesia 32(1):133-147 · 2014 Energy from sonication, physical manipulation (such as extrusion by forcing MLV dispersions through filters with fixed pore sizes), or some other high energy mechanism is required to convert multilayered bilayer assemblies into unilamellar vesicles. If the MLVs contain a membrane protein then you should worry about whether the protein will survive these procedures in folded and functional form. Vesicles can also be prepared by dissolving lipids using detergents and then removing the detergent using BioBeads-SM dialysis, size exclusion chromatography or by diluting the solution to below the detergent’s critical micelle concentration. These are much gentler methods that a membrane protein may well survive with intact structure and function. From: Avanti Polar Lipids Catalog Bilayers can undergo phase transitions at a critical temperature, Tm. Native bilayers are usually in the fluid (liquid crystalline) phase. -

Chemical Disinfectant and Cleaning Agent Safety – Frequently Asked Questions
Caribbean Public Health Agency Technical Guidance: COVID-19 Series No 33 Chemical Disinfectant and Cleaning Agent Safety – Frequently Asked Questions Suggested Citation: CARPHA. (2020). Chemical disinfectant and cleaning agent safety – frequently asked questions. Caribbean Public Health Agency Technical Guidance: COVID-19 Series, no. 33. CARPHA 2020 Chemical Disinfectant and Cleaning Agent Safety – Frequently Asked Questions 9 July 2020 Why are we concerned about cleaning agents and disinfectants? Selecting the correct chemical agent for cleaning and disinfecting and safely handling and using that agent is essential to health and wellbeing. Recently, with the increased focus on cleaning to reduce the risk of exposure to coronavirus disease, there have been an associated increase in media stories warning the public of the dangerous chemicals used for disinfectants. All chemical cleaning agents can be harmful to the health of the person using the product if it is not used as directed on the label by the manufacturer. Chemical agents can cause burns, breathing difficulties or damage to the lungs, or possibly result in poisoning. Persons should carefully read instructions for use, safety data sheets, and any health warnings to ensure they have selected the correct chemical agents, have the appropriate personal protection, and use and store the chemicals in a safe way. What are the properties of effective chemical cleaning agents and disinfectants? A wide variety of chemical agents can be used for cleaning, sanitising, and disinfecting. There are 4 categories of cleaners: detergents, degreasers, abrasives, and acids. Each is effective for cleaning different surfaces depending on the type of soiling. Different chemical agents are effective for killing different bacteria and viruses.1 Molds and fungus will require a sporicidal agent2. -

The Biomatic® Parts Cleaning System Is…
BIOMATIC® The Environmentally Friendly Bioremediation Parts Washer 1 The Biomatic® Parts Cleaning System Is… • A Bioremediation System. The Graymills Biomatic Parts Cleaning System uses the natural technology of microbial bioremediation to reduce oils, greases and other hydrocarbons introduced into the cleaning system to water and carbon dioxide (CO2). No oil and hydrocarbon buildup means the unit continues to clean parts indefinitely (we recommend cleaning the tank once a year, but the fluid can be put back in for continued use if desired.) • A Waste Minimization System. Talking to your customers about “waste minimization” will get their attention. With the Graymills Biomatic Parts Cleaning System, users will go from monthly dumping of fluid and unit cleanup…to once a year. That’s a “waste minimization” sales story few customers can resist. • A Tough Cleaner. Aqueous based Super Biotene® is pH neutral with a mild, pleasant odor. A mixture of emulsifiers and surfactants, they contain no VOCs, so they’re EPA and air quality friendly! Super Biotene cleans grease and grime off even the dirtiest parts, then the microbes go to work, reducing the hydrocarbon contaminants (oils, greases) to water and CO2. • A Good Investment. Recycling costs are slashed…parts cleaner waste is minimized…the dirtiest parts come clean…and the system is operator friendly. 2 How The Biomatic® Parts Cleaning System Works It is important to understand that the Biomatic® Parts Cleaning System is more than individual products - it is a total cleaning environment. The Biomatic Parts Cleaning System relies on the perfect balance between the Super Biotene® cleaning solution and the microbes introduced into the system. -
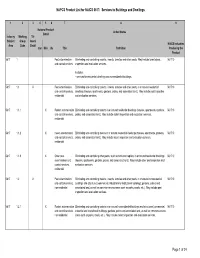
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings
NAPCS Product List for NAICS 5617: Services to Buildings and Dwellings 1 2 3 456 7 8 9 National Product United States Detail Industry Working Tri- Subject Group lateral NAICS Industries Area Code Detail Can Méx US Title Definition Producing the Product 5617 1 Pest extermination Eliminating and controlling rodents, insects, termites and other pests. May include termite/pest 561710 and control services inspection and evaluation services. Includes: • services for residential dwellings and nonresidential buildings. 5617 1.1 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around residential 561710 and control services, dwellings (houses, apartments, gardens, patios, and associated land). May include pest inspection residential and evaluation services. 5617 1.1.1 X Rodent extermination Eliminating and controlling rodents in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include rodent inspection and evaluation services. residential 5617 1.1.2 X Insect extermination Eliminating and controlling insects in or around residential dwellings (houses, apartments, gardens, 561710 and control services, patios, and associated land). May include insect inspection and evaluation services. residential 5617 1.1.9 X Other pest Eliminating and controlling other pests. such as birds and reptiles, in or around residential dwellings 561710 extermination and (houses, apartments, gardens, patios, and associated land). May include other pest inspection and control services, evaluation services. residential 5617 1.2 X Pest extermination Eliminating and controlling rodents, insects, termites and other pests, in or around nonresidential 561710 and control services, buildings and structures (commercial, industrial and institutional buildings; gardens, patios and nonresidential associated land, as well as common-access areas such as parks, roads, etc.). -

We Take Standards for Hygiene and Cleanliness Very Seriously and Are Taking Additional Steps to Ensure the Safety of Our Guests and Associates
We take standards for hygiene and cleanliness very seriously and are taking additional steps to ensure the safety of our guests and associates. On a daily basis, associates at our hotel, villas and homes, clubhouses, nature center and other public areas are working to ensure that they meet the latest guidance on hygiene and cleaning. Our health and safety measures are designed to address a broad spectrum of viruses, including COVID-19, and include everything from frequent handwashing hygiene and cleaning product specifications to guest room and common area cleaning procedures. Specific steps Kiawah Island Golf Resort is taking include: Associate Health, Safety and Knowledge: Resort associates – and their own health, safety and knowledge – are essential to an effective cleaning program. Here are some ways we’re supporting them: • Hand Hygiene: Proper and frequent handwashing is vital to help combat the spread of viruses. In our daily meetings, our teams are reminded that cleanliness starts with this simple act. It’s important for their health and that of our guests. • Ongoing Training: In addition to training on housekeeping and hygiene protocols, resort associates are also completing enhanced COVID-19 awareness training. • Real Time Information: Kiawah Island Golf Resort continues to coordinate with national, regional, state and local authorities, including the Centers for Disease Control and Prevention (CDC) and South Carolina Department of Health and Environmental Control (DHEC) to ensure we are apprised of the latest information. Cleaning Products and Protocols: The resort uses cleaning products and protocols which are effective against viruses, including: • Guest Rooms: The Sanctuary and Resort Villas and Homes use cleaning and disinfecting protocols to clean rooms after guests depart and before the next guest arrives, with particular attention paid to high-touch areas and items. -

VOLUME 2 Cleaning Agents, Sanitisers and Disinfectants in Food Businesses: Detection of Traces and Human Risk Assessment Processes
Chemicals in Food Hygiene VOLUME 2 Cleaning agents, sanitisers and disinfectants in food businesses: detection of traces and human risk assessment processes Chemicals in Food Hygiene – Volume 2 Foreword The Global Food Safety Initiative (GFSI) is a considering carry-over risks of traces in non-profit industry association tasked with foods; promoting continuous improvement of food ➢ Reviewed and identified gaps in the safety management systems to ensure suitability of existing methods for confidence in the delivery of safe food to detection of traces at relevant points in consumers worldwide. GFSI provides a the food production process; platform for collaboration between some of ➢ Developed the GFSI position on the use of the world’s leading food safety experts from cleaning agents, sanitisers and retailer, producers and food service disinfectants and the relationship with companies, service providers associated with microbial resistance. the food supply chain, international organisations, academia and government. The TWG produced 2 volumes within one document: Since GFSI’s inception in 2000, experts from all over the world have been collaborating in ➢ Volume one of this document provides a numerous Technical Working Groups (TWG) high-level overview of the considerations to tackle current food safety issues defined that a food business operator needs to by GFSI stakeholders. In 2017 a TWG was consider in relation to ensuring established to determine best practices in appropriate hygienic practices. This relation to biocides (defined as the residues volume is aimed at a variety of readers from cleaning agents, sanitisers and from the food truck operator or farmer disinfectants) in the food supply chain. -

Cleaning and Sanitizing I. Cleaning 2 Types Of
Cleaning and Sanitizing CLEANING AND SANITIZING I. CLEANING 2 TYPES OF CLEANING COMPOUNDS 2 PROPERTIES OF A CLEANER 2 FACTORS THAT AFFECT CLEANING EFFICIENCY 2 CLEANING OPERATION 3 II. SANITIZING 4 HEAT 4 CHEMICAL SANITIZERS 5 FACTORS AFFECTING SANITIZING 7 III. DISHWASHING MACHINES 8 HOT WATER SANITIZING 8 CHEMICAL SANITIZING 9 REQUIREMENTS FOR A SUCCESSFUL DISHWASHING OPERATION 10 CHECKING A DISHMACHINE 10 COMMON PROBLEMS 12 IV. DEFINITIONS: 14 V. QUIZ 16 VI. ANSWER KEY TO QUIZ 18 VII. REFERENCES: 18 1 Cleaning and Sanitizing This section is presented primarily for information. The only information the BETC participant will be responsible for is to know the sanitization standards for chemical and hot water sanitizing as found in the Rules for Food Establishment Sanitation. CLEANING AND SANITIZING I. CLEANING Cleaning is a process which will remove soil and prevent accumulation of food residues which may decompose or support the growth of disease causing organisms or the production of toxins. Listed below are the five basic types of cleaning compounds and their major functions: 1. Basic Alkalis - Soften the water (by precipitation of the hardness ions), and saponify fats (the chemical reaction between an alkali and a fat in which soap is produced). 2. Complex Phosphates - Emulsify fats and oils, disperse and suspend oils, peptize proteins, soften water by sequestering, and provide rinsability characteristics without being corrosive. 3 Surfactant - (Wetting Agents) Emulsify fats, disperse fats, provide wetting properties, form suds, and provide rinsability characteristics without being corrosive. 4. Chelating - (Organic compounds) Soften the water by sequestering, prevent mineral deposits, and peptize proteins without being corrosive. -

Knolltextiles Cleaning Information
Cleaning Information Regular Maintenance It is important to vacuum upholstery regularly to remove the surface dust that builds up and slowly contributes to a greying effect that diminishes the clarity of the original color. Please note that vacuuming should be done with the proper attachments to avoid breaking down the fibers and contributing to “pilling.” In addition to regular vacuuming, a professional cleaner should come in at least once or twice a year to thoroughly clean the fabrics and remove the soiling that a normal vacuum cleaner cannot reach. The expense of this service is small compared to the reward of seating that looks good as new after a lot of use. Cleaning Codes W: Water-based cleaning agents or foam may be used for cleaning this fabric. S: Only mild, pure water-free dry cleaning solvents may be used for cleaning this fabric. W-S: Water-based cleaning agents and foam or mild, waterfree solvents may be used for cleaning this fabric. W Bleach: Water-based or foam cleaning agents or diluted household bleach may be used for cleaning this fabric. W-S Bleach: Clean with water or solvent-based cleaning agents or diluted household bleach. X: This fabric should be vacuumed or brushed lightly to remove soil. Warning: Do not use water-based foam or liquid cleaning agents of any type on this fabric. Cleaning vs Sanitizing vs Disinfecting Cleaning, disinfecting and sanitizing are often used synonymously, but they are not the same thing. Cleaning removes the visible foreign matter from a surface. Disinfecting, when done according to the instructions of a suitable cleaner, kills all bacteria and viruses present, while sanitizing reduces the level of bacteria and viruses present. -

Safety Data Sheet Quaternary Disinfectant Cleaner
SAFETY DATA SHEET QUATERNARY DISINFECTANT CLEANER SECTION 1. PRODUCT AND COMPANY IDENTIFICATION Product name : QUATERNARY DISINFECTANT CLEANER Other means of identification : Not applicable Recommended use : Disinfectant Restrictions on use : Reserved for industrial and professional use. Product dilution information : 0.39 % - 1.54 % Company : Ecolab Inc. 1 Ecolab Place St. Paul, Minnesota USA 55102 1-800-352-5326 Emergency health : 1-800-328-0026 (US/Canada), 1-651-222-5352 (outside US) information Issuing date : 03/01/2021 SECTION 2. HAZARDS IDENTIFICATION GHS Classification Product AS SOLD Flammable liquids : Category 3 Acute toxicity (Oral) : Category 4 Acute toxicity (Dermal) : Category 4 Skin corrosion : Category 1A Serious eye damage : Category 1 Product AT USE DILUTION Acute toxicity (Dermal) : Category 4 Eye irritation : Category 2B GHS label elements Product AS SOLD Hazard pictograms : Signal Word : Danger Hazard Statements : Flammable liquid and vapor. Harmful if swallowed or in contact with skin. Causes severe skin burns and eye damage. Precautionary Statements : Prevention: Keep away from heat/ sparks/ open flames/ hot surfaces. No smoking. Keep container tightly closed. Ground/bond container and receiving equipment. Use explosion-proof electrical/ ventilating/ lighting/ equipment. Use only non-sparking tools. Take precautionary measures against static discharge. Wash skin thoroughly after handling. Do not eat, drink or smoke when using this product. Wear 913496 1 / 13 SAFETY DATA SHEET QUATERNARY DISINFECTANT CLEANER protective gloves/ protective clothing/ eye protection/ face protection. Response: IF SWALLOWED: Call a POISON CENTER/ doctor if you feel unwell. Rinse mouth. IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. IF ON SKIN (or hair): Take off immediately all contaminated clothing.