Fabrication Via Ultra-Short Pulsed Laser Plasma- Mediated
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Martian Crater Morphology
ANALYSIS OF THE DEPTH-DIAMETER RELATIONSHIP OF MARTIAN CRATERS A Capstone Experience Thesis Presented by Jared Howenstine Completion Date: May 2006 Approved By: Professor M. Darby Dyar, Astronomy Professor Christopher Condit, Geology Professor Judith Young, Astronomy Abstract Title: Analysis of the Depth-Diameter Relationship of Martian Craters Author: Jared Howenstine, Astronomy Approved By: Judith Young, Astronomy Approved By: M. Darby Dyar, Astronomy Approved By: Christopher Condit, Geology CE Type: Departmental Honors Project Using a gridded version of maritan topography with the computer program Gridview, this project studied the depth-diameter relationship of martian impact craters. The work encompasses 361 profiles of impacts with diameters larger than 15 kilometers and is a continuation of work that was started at the Lunar and Planetary Institute in Houston, Texas under the guidance of Dr. Walter S. Keifer. Using the most ‘pristine,’ or deepest craters in the data a depth-diameter relationship was determined: d = 0.610D 0.327 , where d is the depth of the crater and D is the diameter of the crater, both in kilometers. This relationship can then be used to estimate the theoretical depth of any impact radius, and therefore can be used to estimate the pristine shape of the crater. With a depth-diameter ratio for a particular crater, the measured depth can then be compared to this theoretical value and an estimate of the amount of material within the crater, or fill, can then be calculated. The data includes 140 named impact craters, 3 basins, and 218 other impacts. The named data encompasses all named impact structures of greater than 100 kilometers in diameter. -
Relative Ages
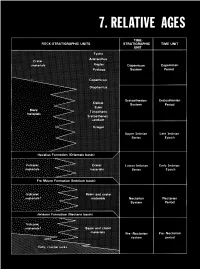
CONTENTS Page Introduction ...................................................... 123 Stratigraphic nomenclature ........................................ 123 Superpositions ................................................... 125 Mare-crater relations .......................................... 125 Crater-crater relations .......................................... 127 Basin-crater relations .......................................... 127 Mapping conventions .......................................... 127 Crater dating .................................................... 129 General principles ............................................. 129 Size-frequency relations ........................................ 129 Morphology of large craters .................................... 129 Morphology of small craters, by Newell J. Fask .................. 131 D, method .................................................... 133 Summary ........................................................ 133 table 7.1). The first three of these sequences, which are older than INTRODUCTION the visible mare materials, are also dominated internally by the The goals of both terrestrial and lunar stratigraphy are to inte- deposits of basins. The fourth (youngest) sequence consists of mare grate geologic units into a stratigraphic column applicable over the and crater materials. This chapter explains the general methods of whole planet and to calibrate this column with absolute ages. The stratigraphic analysis that are employed in the next six chapters first step in reconstructing -

DMAAC – February 1973
LUNAR TOPOGRAPHIC ORTHOPHOTOMAP (LTO) AND LUNAR ORTHOPHOTMAP (LO) SERIES (Published by DMATC) Lunar Topographic Orthophotmaps and Lunar Orthophotomaps Scale: 1:250,000 Projection: Transverse Mercator Sheet Size: 25.5”x 26.5” The Lunar Topographic Orthophotmaps and Lunar Orthophotomaps Series are the first comprehensive and continuous mapping to be accomplished from Apollo Mission 15-17 mapping photographs. This series is also the first major effort to apply recent advances in orthophotography to lunar mapping. Presently developed maps of this series were designed to support initial lunar scientific investigations primarily employing results of Apollo Mission 15-17 data. Individual maps of this series cover 4 degrees of lunar latitude and 5 degrees of lunar longitude consisting of 1/16 of the area of a 1:1,000,000 scale Lunar Astronautical Chart (LAC) (Section 4.2.1). Their apha-numeric identification (example – LTO38B1) consists of the designator LTO for topographic orthophoto editions or LO for orthophoto editions followed by the LAC number in which they fall, followed by an A, B, C or D designator defining the pertinent LAC quadrant and a 1, 2, 3, or 4 designator defining the specific sub-quadrant actually covered. The following designation (250) identifies the sheets as being at 1:250,000 scale. The LTO editions display 100-meter contours, 50-meter supplemental contours and spot elevations in a red overprint to the base, which is lithographed in black and white. LO editions are identical except that all relief information is omitted and selenographic graticule is restricted to border ticks, presenting an umencumbered view of lunar features imaged by the photographic base. -

Scientific Instrument Society
Scientific Instrument Society Bulletin of the Scientific Instrument Society No. 46 September 1995 Bulletin of the Scientific Instrument Society LSSN0956-8271 For Table of Contents, see inside back cover President Gerard Turner Honorary Committee Howard Dawes, Chairman Stuart Talbot, Secretary John Didcock, Treasurer Willem Hackmann, Editor Michael Cowham, Ad~wtising Manager Trevor Waterman, Meetings Secretary Gloria Clifton Jane [nsley Arthur Middleton Alan Morton Membership and Administrative Matters The Executive Officer (Wing Cmdr. Geoffnm] Bennett) 31 High Street Stanford in the Vale Faringdon Tel: 01367 710223 Oxon SN7 8LH Fax: 01367 718963 See inside back cover for information on membership Editorial Matters Dr. Willem D. Hackmann Museum of the History of Science Old Ashmolean Building Tel: 01865 277282 (office) Broad Street Fax: 01865 277288 Oxford OXI 3AZ Tel: 01865 54058 (home) Advertising Mr Michael Cowham The Mount "loft Tel: 01223 263532/262684 Cambridge CB3 7RL Fax: 01223 263948 Organization of Meetings Mr Trevor Waterman 75a Jermyn Street Tel: 0171-930 2954 London SWIY 6NP Fax: 0171-321 0212 Typesetting and Printing Lithoflow Lid 26-36 Wharfdale Road Kings Cross Tel: 0171-833 2344 London NI 9RY Fax: 0171-833 8150 Price: £6 per issue, including back numbers where available. (Please enquire 04 Exec. Officer if sets are required.) The Scientific Instrument Society is Registered Charity No. 326733 © The Scientific lnsVument Society 19')5 Editorial X-ray image of a metal grid taken in THE EM)iF.'.; G.A,ZETTL Crookes' laboratory, but not by him as he -. + .__ was in South Africa when Rontgen's discovery was announced. There is also the metal grid and the X-ray tube used in producing this image. -

Apollo 17 Index: 70 Mm, 35 Mm, and 16 Mm Photographs
General Disclaimer One or more of the Following Statements may affect this Document This document has been reproduced from the best copy furnished by the organizational source. It is being released in the interest of making available as much information as possible. This document may contain data, which exceeds the sheet parameters. It was furnished in this condition by the organizational source and is the best copy available. This document may contain tone-on-tone or color graphs, charts and/or pictures, which have been reproduced in black and white. This document is paginated as submitted by the original source. Portions of this document are not fully legible due to the historical nature of some of the material. However, it is the best reproduction available from the original submission. Produced by the NASA Center for Aerospace Information (CASI) Preparation, Scanning, Editing, and Conversion to Adobe Portable Document Format (PDF) by: Ronald A. Wells University of California Berkeley, CA 94720 May 2000 A P O L L O 1 7 I N D E X 7 0 m m, 3 5 m m, A N D 1 6 m m P H O T O G R A P H S M a p p i n g S c i e n c e s B r a n c h N a t i o n a l A e r o n a u t i c s a n d S p a c e A d m i n i s t r a t i o n J o h n s o n S p a c e C e n t e r H o u s t o n, T e x a s APPROVED: Michael C . -

Odd Year Nur-Rn-Lic-30550 G
LicenseNumber FirstName MiddleName LastName RenewalGroup NUR-LPN-LIC-6174 DOLORES ROSE AABERG 2019 - ODD YEAR NUR-RN-LIC-30550 GRETCHEN ELLEN AAGAARD-SHIVELY 2019 - ODD YEAR NUR-RN-LIC-128118 CAMBRIA LAUREN AANERUD 2019 - ODD YEAR NUR-RN-LIC-25862 SOPHIA SABINA AANSTAD 2018 - EVEN YEAR NUR-APRN-LIC-124944 ERIN EDWARD AAS 2018 - EVEN YEAR NUR-RN-LIC-105371 ERIN EDWARD AAS 2018 - EVEN YEAR NUR-RN-LIC-34536 BRYON AAS 2019 - ODD YEAR NUR-RN-LIC-39208 JULIA LYNN AASEN 2018 - EVEN YEAR NUR-APRN-LIC-130522 LORI ANN AASEN 2019 - ODD YEAR NUR-RN-LIC-130520 LORI ANN AASEN 2019 - ODD YEAR NUR-RN-LIC-21015 DEBBIE ABAR 2018 - EVEN YEAR NUR-APRN-LIC-130757 LUKE G ABAR 2018 - EVEN YEAR NUR-RN-LIC-130756 LUKE GORDON ABAR 2019 - ODD YEAR NUR-RN-LIC-31911 AIMEE KRISTINE ABBOTT 2018 - EVEN YEAR NUR-RN-LIC-29448 DENISE M ABBOTT 2018 - EVEN YEAR NUR-RN-LIC-131150 SARAH FRANCES ABBOTT 2018 - EVEN YEAR NUR-LPN-LIC-31701 ANGIE ABBOTT 2019 - ODD YEAR NUR-LPN-LIC-33325 HEIDI ABBOTT 2019 - ODD YEAR NUR-LPN-LIC-4920 LORI ANN ABBOTT 2019 - ODD YEAR NUR-LPN-LIC-97426 DAYMON ABBOTT Expired - 2018 - EVEN YEAR NUR-RN-LIC-13260 ROBERT C ABBOTT Expired - 2018 - EVEN YEAR NUR-RN-LIC-17858 MONICA MAY ABDALLAH 2018 - EVEN YEAR NUR-RN-LIC-48890 STEVEN P ABDALLAH 2019 - ODD YEAR NUR-APRN-LIC-101391 LANEICE LORRAINE ABDEL-SHAKUR Expired - 2018 - EVEN YEAR NUR-RN-LIC-101333 LANEICE LORRAINE ABDEL-SHAKUR Expired - 2018 - EVEN YEAR NUR-RN-LIC-96606 RENDI L ABEL 2018 - EVEN YEAR NUR-RN-LIC-97338 LAURA ANN ABEL 2019 - ODD YEAR NUR-RN-LIC-69876 LACEY ANN ABELL 2019 - ODD YEAR NUR-RN-LIC-131932 -

Experimentally Demonstrated the Intrinsic Instability of the Fluorite-Type Zr Cation Network
0246110 Rta(i) 1 1,2 l,4 1,6 1,l 2 22 Rta(i) Figure 2. Temperature dependence of Zr-FTfor pure orthorhombic zirconia Figure 3 Temperature dependence of Zr-0 shell in tetragonal zirconia solid solution Our study has experimentally demonstrated the intrinsic instability of the fluorite-type Zr cation network. Coherent scattering beween Conclusions central Zr ion and distant cations is weaker and vibration modes of the Zr-cation network are It is found that, for all of zirconia solid softer in tetragonal zirconia than in monoclmic, solutions studied, the dopant-oxygen distances are orthorhombic, or stabilized cubic zirconia. In si@icantly ddferent from the Zr-0 distances. addition, the outer foux oxygens in the 8-fold The dopant-cation distances, on the other hand, coordinated Zr-0 polyhedron are only loosely are usually very close to the Zr-Zr distance, solutions. A bonded and are subject to very large static and confirming the formation of solid dynamic distortions. This effect is illustrated by general structural picture for zirconia solid the expanded portion of the Fourier transform solutions is one that places the dopant cations shown in Figure 3. randomly on tbe Zr sites in the cation network, but with distorted and sometimes very different Dopant Structure dopant-oxygen polyhedra surrounding these dopants. In the case of Ge4+ doping and Y-Nb We have also successfully performed co-doping, short-rangecation ordering has been EXAFS experiments at the dopant absorption suggested from our EXAFS results. edges for doped zirconia solid solutions. Dopants Based on the above structural information studied include the Ce-,Nb-, and Y-K edges as and other EXAFS results obtained from NSLS, well as Ce-LIn edge. -

The Lateral Growth and Coalesence of Magma Systems Royalsocietypublishing.Org/Journal/Rsta Juliet Biggs1 and Catherine Annen1,2
The lateral growth and coalesence of magma systems royalsocietypublishing.org/journal/rsta Juliet Biggs1 and Catherine Annen1,2 1School of Earth Sciences, University of Bristol, Queen’s Road, Bristol, BS8 1RJ, UK Research 2Univ. Grenoble Alpes, Univ. Savoie Mont Blanc, CNRS, IRD, IFSTTAR, Cite this article: Biggs J, Annen C. 2019 The ISTerre, 38000 Grenoble, France lateral growth and coalesence of magma JB, 0000-0002-4855-039X systems . Phil.Trans.R.Soc.A377: 20180005. http://dx.doi.org/10.1098/rsta.2018.0005 Thermal and mechanical models of magma reservoir growth need to be reconciled with deformation patterns and structural relationships observed at Accepted: 16 October 2018 active magma systems. Geophysical observations provide a series of short time-scale snap-shots (100– One contribution of 15 to a Theo Murphy 102 years) of the long-term growth of magmatic 3 6 meeting issue ‘Magma reservoir architecture bodies (10 –10 years). In this paper, we first review evidence for the growth of magmatic systems along and dynamics’. structural features and the associated deformation patterns. We then define three distinct growth stages, Subject Areas: (1) aligned melt pockets, (2) coalesced reservoirs, (3) volcanology, geophysics highly evolved systems, which can be distinguished using short-term surface observations. We use Keywords: two-dimensional thermal models to provide first- thermal models, surface deformation, order constraints on the time scales and conditions transcrustal magmatic systems associated with coalescence of individual magma bodies into large-scale reservoirs. We find that closely spaced intrusions (less than 1 km apart) can develop Author for correspondence: combined viscoelastic shells over time scales of 10s kyr Juliet Biggs and form laterally extensive mush systems over time e-mail: [email protected] scales of 10–100 kyr. -

The Case for Rainfall on a Warm, Wet Early Mars Robert A
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 107, NO. E11, 5111, doi:10.1029/2001JE001505, 2002 The case for rainfall on a warm, wet early Mars Robert A. Craddock Center for Earth and Planetary Studies, National Air and Space Museum, Smithsonian Institution, Washington, District of Columbia, USA Alan D. Howard Department of Environmental Sciences, University of Virginia, Charlottesville, Virginia, USA Received 11 April 2001; revised 10 April 2002; accepted 10 June 2002; published 23 November 2002. [1] Valley networks provide compelling evidence that past geologic processes on Mars were different than those seen today. The generally accepted paradigm is that these features formed from groundwater circulation, which may have been driven by differential heating induced by magmatic intrusions, impact melt, or a higher primordial heat flux. Although such mechanisms may not require climatic conditions any different than today’s, they fail to explain the large amount of recharge necessary for maintaining valley network systems, the spatial patterns of erosion, or how water became initially situated in the Martian regolith. In addition, there are no clear surface manifestations of any geothermal systems (e.g., mineral deposits or phreatic explosion craters). Finally, these models do not explain the style and amount of crater degradation. To the contrary, analyses of degraded crater morphometry indicate modification occurred from creep induced by rain splash combined with surface runoff and erosion; the former process appears to have continued late into Martian history. A critical analysis of the morphology and drainage density of valley networks based on Mars Global Surveyor data shows that these features are, in fact, entirely consistent with rainfall and surface runoff. -

Upper Darby High School, 601 N Lansdowne Ave, Drexel Hill, PA
Basalt Thickness of Mare Tranquillitatis using Two Methods Upper Darby High School, 601 N Lansdowne Ave, Drexel Hill, PA Gutama Biru, Chris DeMott, Galen Farmer, Daniel Gordon, Isabel Hunt, Kenneth Lin, Thomas Nguyen, Zach Thornton, Vince Tran, Most Yeasmin PROBLEM ISOPACH MAPS The purpose of this experiment is to evaluate two methods of calculating basalt thickness in Mare Tranquillitatis. Figure 1 is the original isopach map used for reference. Figures 2 and 3 are isopach maps made using 3DField software. INTRODUCTION Lunar maria are large impact craters or basins that have been filled with basalt. While the Moon was still cooling, lava seeped into these basins through cracks, cooling to form the maria. This study compares two methods of determining the thickness of basalt in Mare Tranquillitatis: the Pre-Mare Crater Method and the Post- Figure 2. Pre-Mare Crater Isopach Mare Crater Method. The first method uses craters from before the mare was formed and the latter uses craters from after the mare was formed, as the names imply. Previous work ( De HONs map reference) has been conducted using these methods to Figure 1. Reference Map (DeHon 1974) calculate mare thickness; however the tools used to collect that data are outdated. The current study, conducted using data from the Lunar Reconnaissance Orbiter and Clementine camera, compares the two methods for determining mare thickness. The Pre-Mare Crater Method used craters that were formed inside the large impact basins before they filled with lava. By measuring the diameter of these craters, the original rim height can be estimated using a relationship defined by Pike (1974, 1977). -

Topographic Characterization of Lunar Complex Craters Jessica Kalynn,1 Catherine L
GEOPHYSICAL RESEARCH LETTERS, VOL. 40, 38–42, doi:10.1029/2012GL053608, 2013 Topographic characterization of lunar complex craters Jessica Kalynn,1 Catherine L. Johnson,1,2 Gordon R. Osinski,3 and Olivier Barnouin4 Received 20 August 2012; revised 19 November 2012; accepted 26 November 2012; published 16 January 2013. [1] We use Lunar Orbiter Laser Altimeter topography data [Baldwin 1963, 1965; Pike, 1974, 1980, 1981]. These studies to revisit the depth (d)-diameter (D), and central peak height yielded three main results. First, depth increases with diam- B (hcp)-diameter relationships for fresh complex lunar craters. eter and is described by a power law relationship, d =AD , We assembled a data set of young craters with D ≥ 15 km where A and B are constants determined by a linear least and ensured the craters were unmodified and fresh using squares fit of log(d) versus log(D). Second, a change in the Lunar Reconnaissance Orbiter Wide-Angle Camera images. d-D relationship is seen at diameters of ~15 km, roughly We used Lunar Orbiter Laser Altimeter gridded data to coincident with the morphological transition from simple to determine the rim-to-floor crater depths, as well as the height complex craters. Third, craters in the highlands are typically of the central peak above the crater floor. We established deeper than those formed in the mare at a given diameter. power-law d-D and hcp-D relationships for complex craters At larger spatial scales, Clementine [Williams and Zuber, on mare and highlands terrain. Our results indicate that 1998] and more recently, Lunar Orbiter Laser Altimeter craters on highland terrain are, on average, deeper and have (LOLA) [Baker et al., 2012] topography data indicate that higher central peaks than craters on mare terrain. -

Geology of Shackleton Crater and the South Pole of the Moon Paul D
GEOPHYSICAL RESEARCH LETTERS, VOL. 35, L14201, doi:10.1029/2008GL034468, 2008 Geology of Shackleton Crater and the south pole of the Moon Paul D. Spudis,1 Ben Bussey,2 Jeffrey Plescia,2 Jean-Luc Josset,3 and Ste´phane Beauvivre4 Received 28 April 2008; revised 10 June 2008; accepted 16 June 2008; published 18 July 2008. [1] Using new SMART-1 AMIE images and Arecibo and details new results on the lighting conditions of the poles Goldstone high resolution radar images of the Moon, we [e.g., Bussey et al., 2008]. investigate the geological relations of the south pole, including the 20 km-diameter crater Shackleton. The south 2. Geological Setting of the South Pole pole is located inside the topographic rim of the South Pole- Aitken (SPA) basin, the largest and oldest impact crater on [3] The dominant feature that influences the geology and the Moon and Shackleton is located on the edge of an topography of the south polar region is the South Pole- interior basin massif. The crater Shackleton is found to be Aitken (SPA) basin, the largest and oldest impact feature on older than the mare surface of the Apollo 15 landing site the Moon [e.g., Wilhelms, 1987]. This feature is over 2600 (3.3 Ga), but younger than the Apollo 14 landing site km in diameter, averages 12 km depth, and is approximately (3.85 Ga). These results suggest that Shackleton may have centered at 56° S, 180° [Spudis et al., 1994]. The basin collected extra-lunar volatile elements for at least the last formed sometime after the crust had solidified and was rigid 2 billion years and is an attractive site for permanent human enough to support significant topographic expression; thus, presence on the Moon.