Groundwater Quality in the Bist-Doab Catchment, Punjab, India: a Pilot Study Comparing Shallow and Deep Aquifers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Visualizing Hydropower Across the Himalayas: Mapping in a Time of Regulatory Decline
HIMALAYA, the Journal of the Association for Nepal and Himalayan Studies Volume 34 Number 2 Article 9 December 2014 Visualizing Hydropower Across the Himalayas: Mapping in a time of Regulatory Decline Kelly D. Alley Auburn University, [email protected] Ryan Hile University of Utah Chandana Mitra Auburn University Follow this and additional works at: https://digitalcommons.macalester.edu/himalaya Recommended Citation Alley, Kelly D.; Hile, Ryan; and Mitra, Chandana. 2014. Visualizing Hydropower Across the Himalayas: Mapping in a time of Regulatory Decline. HIMALAYA 34(2). Available at: https://digitalcommons.macalester.edu/himalaya/vol34/iss2/9 This work is licensed under a Creative Commons Attribution 3.0 License. This Research Article is brought to you for free and open access by the DigitalCommons@Macalester College at DigitalCommons@Macalester College. It has been accepted for inclusion in HIMALAYA, the Journal of the Association for Nepal and Himalayan Studies by an authorized administrator of DigitalCommons@Macalester College. For more information, please contact [email protected]. Visualizing Hydropower Across the Himalayas: Mapping in a time of Regulatory Decline Acknowledgements Earlier drafts of this paper were presented at the BAPA-BEN International Conference on Water Resources in Dhaka, Bangladesh in 2013 and for the AAA panel on Developing the Himalaya in 2012. The authors appreciate the comments and support provided by members who attended these sessions. Our mapping project has been supported by the College of Liberal Arts and the Center for Forest Sustainability at Auburn University. This research article is available in HIMALAYA, the Journal of the Association for Nepal and Himalayan Studies: https://digitalcommons.macalester.edu/himalaya/vol34/iss2/9 Visualizing Hydropower across the Himalayas: Mapping in a time of Regulatory Decline Kelly D. -

National Ganga River Basin Authority (Ngrba)
NATIONAL GANGA RIVER BASIN AUTHORITY (NGRBA) Public Disclosure Authorized (Ministry of Environment and Forests, Government of India) Public Disclosure Authorized Environmental and Social Management Framework (ESMF) Public Disclosure Authorized Volume I - Environmental and Social Analysis March 2011 Prepared by Public Disclosure Authorized The Energy and Resources Institute New Delhi i Table of Contents Executive Summary List of Tables ............................................................................................................... iv Chapter 1 National Ganga River Basin Project ....................................................... 6 1.1 Introduction .................................................................................................. 6 1.2 Ganga Clean up Initiatives ........................................................................... 6 1.3 The Ganga River Basin Project.................................................................... 7 1.4 Project Components ..................................................................................... 8 1.4.1.1 Objective ...................................................................................................... 8 1.4.1.2 Sub Component A: NGRBA Operationalization & Program Management 9 1.4.1.3 Sub component B: Technical Assistance for ULB Service Provider .......... 9 1.4.1.4 Sub-component C: Technical Assistance for Environmental Regulator ... 10 1.4.2.1 Objective ................................................................................................... -

Study of Groundwater Recharge in Rechna Doab Using Isotope Techniques
\ PINSTECH/RIAD-133 STUDY OF GROUNDWATER RECHARGE IN RECHNA DOAB USING ISOTOPE TECHNIQUES M. ISHAQ SAJJAD M. AZAM TASNEEM MANZOOR AHMAD S. D. HUSSAIN IQBAL H. KHAN WAHEED AKRAM RADIATION AND ISOTOPE APPLICATIONS DIVISION Pakistan Institute of Nuclear Science and Technology P. O. Nilore, Islamabad June, 1992 PINSTECH/RIAD-133 STUDY OF GROUNDWATER RECHARGE IN RECHNA DOAB USING ISOTOPE TECHNIQUES M. ISHAQ SAJJAD M. AZAM TASNEEM MANZOOR AHMAD S. D. HUSSAIN IQBAL H. KHAN WAHEED AKRAM RADIATION AND ISOTOPE APPLICATIONS DIVISION PAKISTAN INSTITUTE OF NUCLEAR SCIENCE AND TECHNOLOGY P. 0. NILORE, ISLAMABAD. June, 1992 CONTENTS ABSTRACT INTRODUCTION THE PROJECT AREA 2.1 General Description of The Area 2.2 Climate 2.3 Surface and Subsurface Geology FIELD WORK LABORATORY WORK RESULTS AND DISCUSSION 5.1 Sources of Groundwater Recharge 5.1.1 Isotopic Data of River/Canal System 5.1.1.1 River Chenab 5.1.1.2 River Ravi 5.1.1.3 Upper Chenab Canal (UCC) 5.1.1.4 Lower Chenab Canal (LCC) 5.1.2 Isotopic Data of Rains ISOTOPIC VARIATIONS IN GROUNDWATER 6.1 Some Features of SD-S^O Diagrams 6.2 Spatial and Temporal Variations of Isotopic Data THE GROUNDWATER RECHARGE FROM DIFFERENT INPUT SOURCES TURN-OVER TIMES OF THE GROUNDWATER VERTICAL DISTRIBUTION OF ISOTOPES CONCLUSIONS ACKNOWLEDGMENTS REFERENCES \ ABSTRACT Isotopic studies were performed in the Rechna Doab area to understand the recharge mechanism, investigate the relative contributions from various sources such as rainfall, rivers and canal system and to estimate the turn-over times and replenishment rate of groundwater. The isotopic data suggest that the groundwater in the project area, can be divided into different zones each having its own characteristic isotopic composition. -

Pincode Officename Statename Minisectt Ropar S.O Thermal Plant
pincode officename districtname statename 140001 Minisectt Ropar S.O Rupnagar PUNJAB 140001 Thermal Plant Colony Ropar S.O Rupnagar PUNJAB 140001 Ropar H.O Rupnagar PUNJAB 140101 Morinda S.O Ropar PUNJAB 140101 Bhamnara B.O Rupnagar PUNJAB 140101 Rattangarh Ii B.O Rupnagar PUNJAB 140101 Saheri B.O Rupnagar PUNJAB 140101 Dhangrali B.O Rupnagar PUNJAB 140101 Tajpura B.O Rupnagar PUNJAB 140102 Lutheri S.O Ropar PUNJAB 140102 Rollumajra B.O Ropar PUNJAB 140102 Kainaur B.O Ropar PUNJAB 140102 Makrauna Kalan B.O Rupnagar PUNJAB 140102 Samana Kalan B.O Rupnagar PUNJAB 140102 Barsalpur B.O Ropar PUNJAB 140102 Chaklan B.O Rupnagar PUNJAB 140102 Dumna B.O Ropar PUNJAB 140103 Kurali S.O Mohali PUNJAB 140103 Allahpur B.O Mohali PUNJAB 140103 Burmajra B.O Rupnagar PUNJAB 140103 Chintgarh B.O Rupnagar PUNJAB 140103 Dhanauri B.O Rupnagar PUNJAB 140103 Jhingran Kalan B.O Rupnagar PUNJAB 140103 Kalewal B.O Mohali PUNJAB 140103 Kaishanpura B.O Rupnagar PUNJAB 140103 Mundhon Kalan B.O Mohali PUNJAB 140103 Sihon Majra B.O Rupnagar PUNJAB 140103 Singhpura B.O Mohali PUNJAB 140103 Sotal B.O Rupnagar PUNJAB 140103 Sahauran B.O Mohali PUNJAB 140108 Mian Pur S.O Rupnagar PUNJAB 140108 Pathreri Jattan B.O Rupnagar PUNJAB 140108 Rangilpur B.O Rupnagar PUNJAB 140108 Sainfalpur B.O Rupnagar PUNJAB 140108 Singh Bhagwantpur B.O Rupnagar PUNJAB 140108 Kotla Nihang B.O Ropar PUNJAB 140108 Behrampur Zimidari B.O Rupnagar PUNJAB 140108 Ballamgarh B.O Rupnagar PUNJAB 140108 Purkhali B.O Rupnagar PUNJAB 140109 Khizrabad West S.O Mohali PUNJAB 140109 Kubaheri B.O Mohali PUNJAB -

Physical Geography of the Punjab
19 Gosal: Physical Geography of Punjab Physical Geography of the Punjab G. S. Gosal Formerly Professor of Geography, Punjab University, Chandigarh ________________________________________________________________ Located in the northwestern part of the Indian sub-continent, the Punjab served as a bridge between the east, the middle east, and central Asia assigning it considerable regional importance. The region is enclosed between the Himalayas in the north and the Rajputana desert in the south, and its rich alluvial plain is composed of silt deposited by the rivers - Satluj, Beas, Ravi, Chanab and Jhelam. The paper provides a detailed description of Punjab’s physical landscape and its general climatic conditions which created its history and culture and made it the bread basket of the subcontinent. ________________________________________________________________ Introduction Herodotus, an ancient Greek scholar, who lived from 484 BCE to 425 BCE, was often referred to as the ‘father of history’, the ‘father of ethnography’, and a great scholar of geography of his time. Some 2500 years ago he made a classic statement: ‘All history should be studied geographically, and all geography historically’. In this statement Herodotus was essentially emphasizing the inseparability of time and space, and a close relationship between history and geography. After all, historical events do not take place in the air, their base is always the earth. For a proper understanding of history, therefore, the base, that is the earth, must be known closely. The physical earth and the man living on it in their full, multi-dimensional relationships constitute the reality of the earth. There is no doubt that human ingenuity, innovations, technological capabilities, and aspirations are very potent factors in shaping and reshaping places and regions, as also in giving rise to new events, but the physical environmental base has its own role to play. -

Ganga As Perceived by Some Ganga Lovers Mother Ganga's Rights Are Our Rights
Ganga as Perceived by Some Ganga Lovers Mother Ganga’s Rights Are Our Rights Pujya Swami Chidanand Saraswati Nearly 500 million people depend every day on the Ganga and Her tributaries for life itself. Like the most loving of mothers, She has served us, nourished us and enabled us to grow as a people, without hesitation, without discrimination, without vacation for millennia. Regardless of what we have done to Her, the Ganga continues in Her steady fl ow, providing the waters that offer nourishment, livelihoods, faith and hope: the waters that represents the very life-blood of our nation. If one may think of the planet Earth as a body, its trees would be its lungs, its rivers would be its veins, and the Ganga would be its very soul. For pilgrims, Her course is a lure: From Gaumukh, where she emerges like a beacon of hope from icy glaciers, to the Prayag of Allahabad, where Mother Ganga stretches out Her glorious hands to become one with the Yamuna and Saraswati Rivers, to Ganga Sagar, where She fi nally merges with the ocean in a tender embrace. As all oceans unite together, Ganga’s reach stretches far beyond national borders. All are Her children. For perhaps a billion people, Mother Ganga is a living goddess who can elevate the soul to blissful union with the Divine. She provides benediction for infants, hope for worshipful adults, and the promise of liberation for the dying and deceased. Every year, millions come to bathe in Ganga’s waters as a holy act of worship: closing their eyes in deep prayer as they reverently enter the waters equated with Divinity itself. -
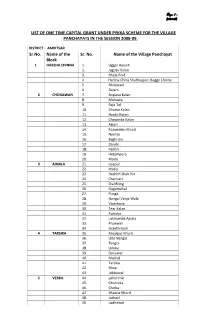
List of One Time Capital Grant Under Pykka Scheme for the Village Panchayats in the Session 2008‐09
Page- 1 - (2008-09) LIST OF ONE TIME CAPITAL GRANT UNDER PYKKA SCHEME FOR THE VILLAGE PANCHAYATS IN THE SESSION 2008‐09. DISTRICT ‐ AMRITSAR Sr.No. Name of the Sr. No. Name of the Village Panchayat Block 1 HARSHA CHINNA 1. Uggar Aulakh 2. Jagdev Kalan 3. Bhala Pind 4. Harsha China Shahbajpur, Bagge Chinne 5. Bhitewad 6. Dalam 2 CHOGAWAN 7. Boparai Kalan 8. Mohawa 9. Raja Tal 10. Dhanoi Kalan 11. Hardo Ratan 12. Chawinda Kalan 13. Attari 14. Rorawalan Khurd 15. Neshta 16. Baghrian 17. Dande 18. Padhri 19. Hetampura 20. Mode 3 AJNALA 21. Issapur 22. Matia 23. Hashim Shah Pur 24. Chamiari 25. Dial Bring 26. Gagomahal 27. Punga 28. Nangal Vanja Wala 29. Viosshova 30. Tear Kalan 31. Ramdas 32. Lakhuwala Ajnala 33. Phoewali 34. Granth Garh 4 TARSIKA 35. Rasulpur Khurd 36. Udo Nangal 37. Tangra 38. Udoke 39. Dariywal 40. Mushal 41. Tarsika 42. Bhoe 43. Jabbowal 5 VERKA 44. Lahorimal 45. Gharinda 46. Chicha 47. Bhakna Khurd 48. Jathaul 49. Lodhewal Page- 2 - (2008-09) 50. Kallewal 51. Sanghna 52. Wadala Bhitewad 6 RAYYA 53. Dera Baba Jaimal Singh 54. Baba Bakala 55. Wadala Kalan 56. Butala 57. Sathiala 58. Gaggar Bhana 59. Butter Kalan 60. Khilchian 61. Umra Nangal 62. Pheruman 7 JANDIALA 63. Bundala GURU 64. Manawala 65. Fathpur Rajputan 66. Mallian 67. Taragarh 68. Chappa Ram Singh 69. Gehri 70. Chattiwind 71. Varpal 8 MAJITHA 72. Majitha 73. Sohian Kalan 74. Kotla Sultan Singh 75. Chawinda Devi 76. Bhangali Kalan 77. Kairon Nangal 78. Wadala 79. Jyantipura 80. -

Covid Positivity Rate Drops Down to 3.5 in Himachal
WWW.YUGMARG.COM REGD NO. CHD/0061/2006-08 | RNI NO. 61323/95 Wednesday June 16, 2021 CHANDIGARH, VOL. XXVI, NO. 137 PAGES 12, RS. 2 YOUR REGION, YOUR PAPER Govt empowers CM asks expert CM flags off sewer WTC final: Ball dealers, firms for panel to study jetting and will swing even litter picking registration of fully effectiveness without saliva, machines says Ishant built-up new vehicles of vaccines in context of new variants PAGE 3 PAGE 4 PAGE 5 PAGE 11 Hry Cabinet gives nod Cinemas, restaurants, gyms to frame Consumer Protection Rules allowed to open in Punjab AGENCY Rajasthan to CHANDIGARH, JUN 15 Okays various set up Vedic SHASHI PAL JAIN In a bid to govern the salary, al- social security CHANDIGARH, JUNE 15: lowances and other service condi- education India reports tions of Presidents and Members of schemes With the state’s Covid positivity 60,471 new State Consumer Disputes Redressal CHANDIGARH: The Haryana board soon rate coming down to 2%, Punjab Commission and District Consumer Cabinet on Tuesday gave its JAIPUR: The Rajasthan Chief Minister Captain Amarinder Disputes Redressal Commissions in approval to increase the rate of government will soon set COVID-19 cases pension, allowance and finan- up a Vedic Education and Singh on Tuesday announced eas- NEW DELHI: India reported Haryana, new Rules cial assistance under the vari- Sanskar Board to revive ing of the restrictions, allowing 60,471 new COVID-19 cases, namely Haryana Con- ous social security schemes the knowledge of Sanskrit restaurants and other eating joints, the lowest count after 75 days, sumer Protection with effect from April 1, 2021. -

Sgd Srf*{A Fxfrfrr{R Fssqr (Fr: Fu:). Irfrrs I 1T-Srsr=*Sfxf+Ar E-Dj *Uts-Dx, *Fl-8, Rilxe Ritrrorol
Orders of Station Allotment, English-899 sgd srf*{a fxfrfrr{r fssqr (fr: fu:). irfrrs I 1t-srsr=*sfxF+ar E-dJ *uts-dx, *fl-8, rilxE ritrrorol 3rax f: 20687 a-DPISE-EST20APPT(APPT)12412021-ESTABLISHMENT-2-DPISE flr$:- 2s-o8-2o2r 1.0 frnfa' f€il'ar, rifrrq ts€ Hrrzir aga etC ,ffi fi€fr' Srrti gss Sdrrt' mflar voa' sfr firefi oo-o+ -zozt d ffi3:drd fe-s'fdrm frr fgx gtffs firtffi AE $fle.o'd trm drt dq fi{-3i|}r{r g fgd.dr A Mga http://educationrecruitmentboard.com 3 }r{trBt ffi atfr frt fEtr6. fire"re|t ?rfre=-6' 6 srf d*fir*art 5 furcs ftdt fr fi3 arB rar 2.o ffi' g TT.fr fuqfr fsd.d uiilErrdEr fse Ea il'sdx€ rffi aftarQrfl O @' A 6.€i' H'ril{E gdx'g rf6'Erir Eera. 4tglz- dt +€ ua:- Sr. Registrati Name Father Name Cetegory Allotted School Ncme Edu. Block District No. on No. I 79t50t475 Kamlesh Kaur Harbhajan Dass Handicaped GSSS Dhade Fateh Bullowal Hoshiarpur (Hearing) Singh 2. 79150Q744 Parbhjot'Kaur Balbir Singh General GHS Rampur Garshanker 2 Hoshiarpur 3. 79r s01052 Sundeep Neelam Kumar EWS GSSS Mahilpur Girls Mahilpur I l"loshiarpur Kumar Sharda 4. 79150t 153 Sapna Soni Ram Paul Soni BC GSSS Haibowal Garshanker 2 Hoshiarpur 5. 791s00365 Nisha Rani Bachittar Singh BC .GSSS Talwara Sec Talwara Hoshiarpur One 6. 791 50163 I Shubha Jain Anil Kumar Jain EWS GSSS Meghowal Mahilpur I Hoshiarpur Doaba 7. 79r 50r 638 Rajdeep Kaur Pamjit Singh General GSSS Jaijon Doaba Mahilpur I Hoshiarpur 8. -

Ganga River Basin Planning Assessment Report
Strategic Basin Planning for Ganga River Basin in India Ganga River Basin Planning Assessment Report Final November 2018 Title Ganga River Basin Planning Assessment Report Client Project Reference Pages World Bank and 1220123-002 1220123-002-ZWS-0003 170 Government of India Keywords India, Ganga, Model, Information System, Hydrology, Geohydrology, Water quality, Ecology, Water allocation, Integrated Water Resources Management Summary The World Bank has assigned Deltares and its partners AECOM India and FutureWater to carry out the project “Analytical Work and Technical Assistance to support Strategic Basin Planning for Ganga River Basin in India” in cooperation with the Government of India. The objectives of the project are 1) to strengthen the capacity with respect to strategic basin planning, 2) to develop a set of scenarios for the development of the Ganga basin, 3) to build a strong and accessible knowledge base and 4) to establish a multi-stakeholder engagement process to support strategic basin planning. This report contains: • The scenario and strategy assessment; • The environmental flow assessment; • The groundwater-surface water interaction assessment. This report contributes to project milestone 5 of the project. Reference Bons, C.A. (Ed.), 2018. Ganga River Basin Planning Assessment Report. Main volume and Appendices. Deltares with AECOM and FutureWater for the World Bank and the Government of India, Report 1220123-002-ZWS-0003. State Final Ganga River Basin Planning Assessment Report 1220123-002-ZWS-0003, November 2018, final -

Comparative Physiography of the Lower Ganges and Lower Mississippi Valleys
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1955 Comparative Physiography of the Lower Ganges and Lower Mississippi Valleys. S. Ali ibne hamid Rizvi Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Rizvi, S. Ali ibne hamid, "Comparative Physiography of the Lower Ganges and Lower Mississippi Valleys." (1955). LSU Historical Dissertations and Theses. 109. https://digitalcommons.lsu.edu/gradschool_disstheses/109 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. COMPARATIVE PHYSIOGRAPHY OF THE LOWER GANGES AND LOWER MISSISSIPPI VALLEYS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Geography ^ by 9. Ali IJt**Hr Rizvi B*. A., Muslim University, l9Mf M. A*, Muslim University, 191*6 M. A., Muslim University, 191*6 May, 1955 EXAMINATION AND THESIS REPORT Candidate: ^ A li X. H. R iz v i Major Field: G eography Title of Thesis: Comparison Between Lower Mississippi and Lower Ganges* Brahmaputra Valleys Approved: Major Prj for And Chairman Dean of Gri ualc School EXAMINING COMMITTEE: 2m ----------- - m t o R ^ / q Date of Examination: ACKNOWLEDGMENT The author wishes to tender his sincere gratitude to Dr. Richard J. Russell for his direction and supervision of the work at every stage; to Dr. -

Surface Water/Groundwater Relationship in Chaj Doab
PINSTECH/RIAD-122 . SURFACE WATER/GROUNDWATER RELATIONSHIP IN CHAJ DOAB RADIATION & ISOTOPE APPLICATIONS DIVISION Pakistan Institute of Nuclear Science & Technology P. O. Nilore Islamabad. JULY, 1990 PINSTECH/RIAD-122 SURFACE WATER/GROUNDWATER RELATIONSHIP , IN CHAJ DOAB S. D. HUSSAIN M. I. SAJJAD W.AKRAM M. AHMAD M. RAFIQ J. A. TARIQ RADIATION & ISOTOPE APPLICATIONS DIVISION Pakistan Institate of Nuclear Science & Technology P. O. Nilore Islamabad. JULY, 1990 PIMSTKCH/RIAD-122 SURFACE WATER/GROUNDWATER RELATIONSHIP IN CHAJ DOAB S.D.HOSSAIN M.I.SAJJAD W.AKRAM M.AHMAD M.RAFIQ J.A.TARIQ RADIATION & ISOTOPE APPLICATIONS DIVISION PAKISTAN INSTITOTE OF NUCLEAR SCIENCE & TECHNOLOGY P.O. NILORE, ISLAMABAD JULY-1990 CONTENTS ABSTRACT INTRODUCTION 1 DESCRIPTION OF THE AREA UNDER INVESTIGATION 2 2.1 General 2 2.2 Physiography 2 2.3 Geology 3 2.4 Climate 4 SAMPLING PLAN 5 ANALYSIS 6 ISOTOPIC CHARACTERISATION OF DIFFERENT HATERS 8 5.1 Rainfall 8 5.2 Surface Hater: Rivers and Canals 9 5.3 Groundwater 11 INTERRELATION OF DIFFERENT WATERS 12 6.1 Surface Water/Shallow Groundwater 12 6.2 Shallow Water/Deep Water 14 6.3 Surface Water/Deep Water 15 PROCESS OF SALINIZATION OF GROUNDWATER 16 CONCLUSIONS 17 ACKNOWLEDGEMENTREFERENCES S 1189 ABSTRACT In order to understand the relationship between surface water and groundwater in Chaj Doab, isotopic and chenical studies were undertaken. The data show that north-eantern part of the doab is mainly recharged by raini;. In the rest o* the doab, surface water and groundwater are generally well connected and the recharge is mostly from the rivers either directly or through irrigation channels.