Cirrus® / Zyrtec-D® 5 Mg/120 Mg Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pericardial, Retroperitoneal, and Pleural Fibrosis Induced by Pergolide
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.66.1.79 on 1 January 1999. Downloaded from J Neurol Neurosurg Psychiatry 1999;66:79–81 79 SHORT REPORT Pericardial, retroperitoneal, and pleural fibrosis induced by pergolide S Shaunak, A Wilkins, J B Pilling, D J Dick Abstract 1992, the emergence of motor fluctuations led Three patients with Parkinson’s disease to the introduction of pergolide, and the dose are described who developed pericardial, of this was gradually increased to a maximum retroperitoneal, and pleural fibrosis asso- of 1mg/day. 1n 1994, 2 years after the ciated with pergolide treatment. Surgical introduction of pergolide, the patient devel- intervention was required in all three oped left flank pain with weight loss, and was cases, either to reach a tissue diagnosis or found to have a mild anaemia (haemoglobin for potentially life threatening complica- 10.4 g/dl), with indices suggesting iron defi- tions. Symptoms emerged on average 2 ciency, and an ESR of 40 mm/h. Upper gastro- years after the institution of treatment, intestinal endoscopy and barium enema gave and were suYciently non-specific to cause negative results. Seven months later right sided significant delays in diagnosis in all cases. chest pain and a non-productive cough devel- The erythrocyte sedimentation rate (ESR) oped; investigations confirmed persistent anae- was raised in the two patients in whom it mia, an ESR of 55 mm/h, and bilateral pleural was measured. Serosal fibrosis is a rarely thickening on chest radiography and CT. Lung reported adverse eVect of pergolide treat- function tests showed a reduction in total lung ment, although it is well described with capacity of 36% with no fall in transfer factor, other dopamine agonists. -

Oxytocin Versus Methylergometrine in the Active Management of Third Stage of Labour
Open Journal of Obstetrics and Gynecology, 2014, 4, 666-671 Published Online August 2014 in SciRes. http://www.scirp.org/journal/ojog http://dx.doi.org/10.4236/ojog.2014.411093 Oxytocin versus Methylergometrine in the Active Management of Third Stage of Labour Ajantha Boopathi1*, Sujir Radhakrishnan Nayak2, Arun Rao2, Bharathi Rao2 1Andal Hospital, Cuddalore, India 2Department of Obstetrics and Gynecology, Kasturba Medical College (A Constituent of Manipal University), Mangalore, India Email: *[email protected] Received 19 June 2014; revised 15 July 2014; accepted 10 August 2014 Copyright © 2014 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Objective: To compare the efficacy of Oxytocin versus Methylergometrine in active management of third stage of labour in reducing risk of postpartum hemorrhage. Methods: This study was carried out by randomly assigning into two groups with 150 women in each group. Group 1 included pa- tients who received injection Oxytocin 10 IU intramuscular within one minute of the birth of the baby. Injection Methylergometrine (0.2 mg) was given intravenously at the delivery of anterior shoulder of the baby to women in Group 2. Outcome measures were the duration of third stage, blood loss, pre and post-delivery hematocrit, side effects and incidence of PPH. Statistical analysis was done using Chi square test, Fischers test, Mann Whitney test, and t test. p < 0.05 was consi- dered significant. Results: Mean duration of third stage of labour, mean blood loss, post-delivery fall in hematocrit and need for additional uterotonics were significantly less in the Group 2. -

I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St
2014;22(1):13-18 TCP Translational and Clinical Pharmacology http://dx.doi.org/10.12793/tcp.2014.22.1.13 Clinical Pharmacology Review for Primary Health Care Providers: I. Antihistamines Seunghoon Han* Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul 137-701, Korea *Correspondence: S. Han; Tel: +82-2-2258-7326, Fax: +82-2-2258-7876, E-mail: [email protected] Received 31 May 2014 Primary health care providers play a critical role in maintaining public health, and the appropri- Accepted 31 May 2014 ate use of pharmaceutical products is one of the major parts of their practice. This series of articles, pISSN: 2289-0882 entitled ‘Clinical Pharmacology Review for Primary Health Care Providers,’ is intended to help pri- mary health care providers select more appropriate prescriptions for frequently used drugs based on up-to-date information. We expect that this effort will contribute to improvements in public health and diminish unnecessary drug use. Introduction tion on the H1 receptor.[8] THERAPEU Antihistamines include some of the most frequently prescribed drugs in the primary health care environment for the symp- Generations and Classes tomatic relief of allergic diseases, the common cold, urticaria, Many primary health care providers are well-informed about T and insomnia.[1-5] The importance of antihistamines has been the different ‘generations’ of antihistamines but not about the ICS TU emphasized as the prevalence of target diseases increases.[6,7] different ‘classes’ characterized according to chemical structure. However, the appropriate use, clinical effectiveness, and target [1] This discrepancy seems reasonable because ‘inter-generation’ T populations for prescription of antihistamines are still a matter differences are more prominent than ‘inter-class’ differences. -

Evidence from Horses with Pituitary Pars Intermedia Dysfunction Jessica S
Fortin et al. BMC Veterinary Research (2020) 16:356 https://doi.org/10.1186/s12917-020-02565-3 RESEARCH ARTICLE Open Access Restoring pars intermedia dopamine concentrations and tyrosine hydroxylase expression levels with pergolide: evidence from horses with pituitary pars intermedia dysfunction Jessica S. Fortin1*, Matthew J. Benskey2, Keith J. Lookingland2, Jon S. Patterson1, Erin B. Howey1, John L. Goudreau2,3 and Harold C. Schott II4* Abstract Background: Pituitary pars intermedia dysfunction (PPID) develops slowly in aged horses as degeneration of hypothalamic dopaminergic neurons leads to proliferation of pars intermedia (PI) melanotropes through hyperplasia and adenoma formation. Dopamine (DA) concentrations and tyrosine hydroxylase (TH) immunoreactivity are markedly reduced in PI tissue of PPID-affected equids and treatment with the DA receptor agonist pergolide results in notable clinical improvement. Thus, we hypothesized that pergolide treatment of PPID-affected horses would result in greater DA and TH levels in PI tissue collected from PPID-affected horses versus untreated PPID-affected horses. To test this hypothesis, pituitary glands were removed from 18 horses: four untreated PPID-affected horses, four aged and four young horses without signs of PPID, and six PPID-affected horses that had been treated with pergolide at 2 µg/kg orally once daily for 6 months. DA concentrations and TH expression levels in PI tissues were determined by high performance liquid chromatography with electrochemical detection and Western blot analyses, respectively. Results: DA and TH levels were lowest in PI collected from untreated PPID-affected horses while levels in the pergolide treated horses were similar to those of aged horses without signs of PPID. -

WO 2014/106238 Al 3 July 2014 (03.07.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/106238 Al 3 July 2014 (03.07.2014) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/404 (2006.01) C07D 209/04 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A61P 25/18 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, PCT/US2013/078453 MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 3 1 December 2013 (3 1.12.2013) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (25) Filing Language: English ZW. (26) Publication Language: English (84) Designated States (unless otherwise indicated, for every (30) Priority Data: kind of regional protection available): ARIPO (BW, GH, 61/747,499 31 December 2012 (3 1. 12.2012) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (71) Applicant: FANG, Qun, Kevin [US/US]; 34 Atwood TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Street, Westfield, MA 02482 (US). -

Progress and Prospects of Ergot Alkaloid Research
Progress and Prospects of Ergot Alkaloid Research Joydeep Mukherjee, Miriam Menge Institut für Technische Chemie, Universität Hannover, Callinstr. 3, D-30167 Hannover, Germany E-mail: [email protected] Ergot alkaloids, produced by the plant parasitic fungi Claviceps purpurea are important pharmaceuticals. The chemistry, biosynthesis, bioconversions, physiological controls, and biochemistry have been extensively reviewed by earlier authors.We present here the research done on the organic synthesis of the ergot alkaloids during the past two decades. Our aim is to apply this knowledge to the synthesis of novel synthons and thus obtain new molecules by directed biosynthesis. The synthesis of clavine alkaloids, lysergic acid derivatives, the use of tryptophan as the starting material, the chemistry of 1,3,4,5-tetrahydrobenzo[cd]indoles, and the structure activity relationships for ergot alkaloids have been discussed. Recent advances in the molecular biology and enzymology of the fungus are also mentioned. Application of oxygen vectors and mathematical modeling in the large scale production of the alkaloids are also discussed. Finally, the review gives an overview of the use of modern analytical methods such as capillary electrophoresis and two-dimensional fluorescence spectroscopy. Keywords. Ergot, Alkaloid synthesis, Claviceps, Directed biosynthesis, Bioreactors 1Introduction . 2 2 Chemistry, Bioconversions, and Directed Biosynthesis . 2 2.1 Chemical Synthesis . 3 2.1.1 Chemical Structures . 3 2.1.1.1 Clavine Alkaloids . 3 2.1.1.2 Simple Lysergic Acid Derivatives . 4 2.1.1.3 Ergopeptines . 4 2.1.1.4 Ergopeptams . 5 2.1.2 Synthesis of Clavine Alkaloids and Lysergic Acid Derivatives . 5 2.1.3 Use of Tryptophan as the Starting Material . -

Biased Ligands Differentially Shape the Conformation of The
International Journal of Molecular Sciences Article Biased Ligands Differentially Shape the Conformation of the Extracellular Loop Region in 5-HT2B Receptors Katrin Denzinger, Trung Ngoc Nguyen, Theresa Noonan, Gerhard Wolber and Marcel Bermudez * Institute of Pharmacy, Freie Universität Berlin, Königin-Luise-Strasse 2-4, 14195 Berlin, Germany; [email protected] (K.D.); [email protected] (T.N.N.); [email protected] (T.N.); [email protected] (G.W.) * Correspondence: [email protected] Received: 22 November 2020; Accepted: 18 December 2020; Published: 20 December 2020 Abstract: G protein-coupled receptors are linked to various intracellular transducers, each pathway associated with different physiological effects. Biased ligands, capable of activating one pathway over another, are gaining attention for their therapeutic potential, as they could selectively activate beneficial pathways whilst avoiding those responsible for adverse effects. We performed molecular dynamics simulations with known β-arrestin-biased ligands like lysergic acid diethylamide and ergotamine in complex with the 5-HT2B receptor and discovered that the extent of ligand bias is directly connected with the degree of closure of the extracellular loop region. Given a loose allosteric coupling of extracellular and intracellular receptor regions, we delineate a concept for biased signaling at serotonin receptors, by which conformational interference with binding pocket closure restricts the signaling repertoire of the receptor. Molecular docking studies of biased ligands gathered from the BiasDB demonstrate that larger ligands only show plausible docking poses in the ergotamine-bound structure, highlighting the conformational constraints associated with bias. This emphasizes the importance of selecting the appropriate receptor conformation on which to base virtual screening workflows in structure-based drug design of biased ligands. -

Prascend® (Pergolide Tablets)1 Mg
PRASCEND- pergolide tablet Boehringer Ingelheim Animal Health USA Inc. ---------- Prascend® (pergolide tablets) 1 mg Dopamine receptor agonist for oral use in horses only Caution: Federal law restricts this drug to use by or on the order of a licensed veterinarian. Description: PRASCEND Tablets are rectangular light red colored, half-scored tablets containing 1 mg pergolide, as pergolide mesylate. Pergolide mesylate is a synthetic ergot derivative and is a potent dopamine receptor agonist. The chemical name of pergolide mesylate is 8β-[(Methylthio) methyl]-6- propylergoline monomethanesulfonate. The chemical structure is: Indication: For the control of clinical signs associated with Pituitary Pars Intermedia Dysfunction (Equine Cushing’s Disease) in horses. Dosage and Administration: Administer orally at a starting dose of 2 mcg/kg once daily. Dosage may be adjusted to effect, not to exceed 4 mcg/kg daily. It has been reported that pergolide tablets may cause eye irritation, an irritating smell, or headache when PRASCEND Tablets are split or crushed. PRASCEND Tablets should not be crushed due to the potential for increased human exposure and care should be taken to minimize exposure when splitting tablets. The tablets are scored and the calculated dosage should be provided to the nearest one-half tablet increment (see Table 1). Table 1 Dosing Table Dosage Dosage Body Weight 2 mcg/kg 4 mcg/kg 136 - 340 kg 0.5 tablet 1 tablet (300 - 749 lb) 341 - 567 kg 1 tablet 2 tablets (750 - 1,249 lb) 568 - 795 kg 1.5 tablets 3 tablets (1,250 - 1,749 lb) 796 - 1,022 kg 2 tablets 4 tablets (1,750 - 2,249 lb) Dosing should be titrated according to individual response to therapy to achieve the lowest effective dose. -

WO 2010/099522 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 2 September 2010 (02.09.2010) WO 2010/099522 Al (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 45/06 (2006.01) A61K 31/4164 (2006.01) kind of national protection available): AE, AG, AL, AM, A61K 31/4045 (2006.01) A61K 31/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2010/025725 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 1 March 2010 (01 .03.2010) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, (26) Publication Language: English TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/156,129 27 February 2009 (27.02.2009) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, HELSINN THERAPEUTICS (U.S.), INC. -

FDA Warns That Abuse and Misuse of the Nasal Decongestant Propylhexedrine Causes Serious Harm This Includes Heart and Mental Health Problems Or Death
FDA warns that abuse and misuse of the nasal decongestant propylhexedrine causes serious harm This includes heart and mental health problems or death 3-25-2021 FDA Drug Safety Communication What safety concern is FDA announcing? The U.S. Food and Drug Administration (FDA) is warning that the abuse and misuse of the over- the-counter (OTC) nasal decongestant propylhexedrine can lead to serious harm such as heart and mental health problems. Some of these complications, which include fast or abnormal heart rhythm, high blood pressure, and paranoia, can lead to hospitalization, disability, or death. Reports of individuals abusing and misusing propylhexedrine have increased in recent years. Propylhexedrine is safe and effective when used as directed. What is FDA doing? We are requesting that all manufacturers of OTC propylhexedrine nasal decongestant inhalers consider product design changes that support its safe use. For example, modifying the product to create a physical barrier that would make tampering with the device and abusing the propylhexedrine inside more difficult. In addition, decreasing the amount of medicine the device contains could also reduce the risk of serious side effects if abused or misused. We continue to evaluate this safety issue and will determine if additional FDA actions are needed. What is propylhexedrine and how can it help me? Propylhexedrine is a nasal decongestant that is available OTC in an inhaler. It is used short term to temporarily relieve nasal congestion due to colds, hay fever, or other upper respiratory allergies. It works by reducing swelling and inflammation of the mucous membrane lining of the nose. -
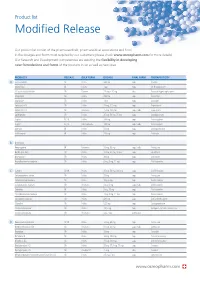
Modified Release
Product list Modified Release Our product list consist of the pharmaceuticals, pharmaceutical associations and food in the dosages and forms most required by our customers (please check www.osmopharm.com for more details). Our Research and Development competencies are assuring the flexibility in developing new formulations and forms of the products in list as well as new ones. PRODUCTS RELEASE BULK FORM DOSAGE FINAL FORM THERAPEUTICITY A Acetazolamide SR Pellets 500 mg caps Diuretic Alfacalcidol IR Pellets 1 μg caps Vit D supplement Alfuzosin Hydrochloride SR Powder 2.5 mg – 10 mg tabs Prostatic Hypertrophy agent Allopurinol SR Pellets 300 mg caps Antiurolytic Alprazolam SR Pellets 1 mg caps Anxiolytic Ambroxol HCI SR Pellets 75 mg, 120 mg caps Expectorant Ambroxol HCI SR Resinates 75 mg, 120 mg caps / tabs Expectorant Amitriptyline SR Pellets 25 mg, 50 mg, 75 mg caps Antidepressant Aspirin EC, IR Pellets 100 mg caps Anticoagulant Aspirin EC, IR Microcapsules 100 mg caps / tabs Anticoagulant Atenolol IR Pellets 50 mg caps Antihypertensive Azithromycin IR Pellets 250 mg caps Antibiotic B B-complex Benproperine IR Resinates 25 mg, 50 mg caps / tabs Antitussive Betahistine 2HCI SR Pellets 12 mg, 24 mg, 48 mg caps Vasodilator Bromopride* SR Pellets 20 mg caps Antiemetic Brompheniramine maleate SR Pellets 6 mg, 8 mg, 12 mg caps Antihistaminic C Caffeine SR, IR Pellets 25 mg, 50 mg, 300 mg caps CNS Stimulant Carbetapentane citrate SR Pellets 75 mg caps Antitussive Carbinoxamine maleate SR Pellets 4mg, 6 mg caps Antihistaminic Carbinoxamine -

Risk Assessment of Argyreia Nervosa
Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos RIVM letter report 2019-0210 Colophon © RIVM 2020 Parts of this publication may be reproduced, provided acknowledgement is given to the: National Institute for Public Health and the Environment, and the title and year of publication are cited. DOI 10.21945/RIVM-2019-0210 W. Chen (author), RIVM L. de Wit-Bos (author), RIVM Contact: Lianne de Wit Department of Food Safety (VVH) [email protected] This investigation was performed by order of NVWA, within the framework of 9.4.46 Published by: National Institute for Public Health and the Environment, RIVM P.O. Box1 | 3720 BA Bilthoven The Netherlands www.rivm.nl/en Page 2 of 42 RIVM letter report 2019-0210 Synopsis Risk assessment of Argyreia nervosa In the Netherlands, seeds from the plant Hawaiian Baby Woodrose (Argyreia nervosa) are being sold as a so-called ‘legal high’ in smart shops and by internet retailers. The use of these seeds is unsafe. They can cause hallucinogenic effects, nausea, vomiting, elevated heart rate, elevated blood pressure, (severe) fatigue and lethargy. These health effects can occur even when the seeds are consumed at the recommended dose. This is the conclusion of a risk assessment performed by RIVM. Hawaiian Baby Woodrose seeds are sold as raw seeds or in capsules. The raw seeds can be eaten as such, or after being crushed and dissolved in liquid (generally hot water).