Appendix A. Survival Strategies of the Ecotonal Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Compendium of Research in the Northwest Territories 2014
Compendium of Research in the Northwest Territories 2014 www.nwtresearch.com This publication is a collaboration between the Aurora Research Institute, the Department of Environment and Natural Resources, Fisheries and Oceans Canada and the Prince of Wales Northern Heritage Centre. Thank you to all who submitted a summary of research or photographs, and helped make this publication possible. Editor: Ashley Mercer Copyright © 2015 ISSN: 1205-3910 Printed by Aurora Research Institute Foreword Welcome to the 2014 Compendium of Research in the Northwest Territories. This year marked a special anniversary for the Aurora Research Institute and northern research. Fifty years ago, the Inuvik Research Laboratory was built and has served as a hub for research in the western arctic ever since. The Lab, as it was known, was first built as an initiative of the Canadian federal government in the newly established community of Inuvik. It remains on the same site today, but in 2011, a new modern multi-purpose facility opened to continue to support research in the north. We have included a brief history of the Lab and its impact in this edition of the Compendium to mark its long lasting importance to many researchers and community members. As part of the 50th anniversary celebration, the Aurora Research Institute team undertook a full set of NWT-wide celebrations. We celebrated the history, capacity and growth of research in the NWT that touched all corners of the territory and beyond. We honoured the significant scientific contributions that have taken place in the NWT over the past 50 years, and the role of NWT researchers, technicians and citizens in these accomplishments. -

Taiga Plains
ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES Taiga Plains Ecosystem Classification Group Department of Environment and Natural Resources Government of the Northwest Territories Revised 2009 ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES TAIGA PLAINS This report may be cited as: Ecosystem Classification Group. 2007 (rev. 2009). Ecological Regions of the Northwest Territories – Taiga Plains. Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, NT, Canada. viii + 173 pp. + folded insert map. ISBN 0-7708-0161-7 Web Site: http://www.enr.gov.nt.ca/index.html For more information contact: Department of Environment and Natural Resources P.O. Box 1320 Yellowknife, NT X1A 2L9 Phone: (867) 920-8064 Fax: (867) 873-0293 About the cover: The small photographs in the inset boxes are enlarged with captions on pages 22 (Taiga Plains High Subarctic (HS) Ecoregion), 52 (Taiga Plains Low Subarctic (LS) Ecoregion), 82 (Taiga Plains High Boreal (HB) Ecoregion), and 96 (Taiga Plains Mid-Boreal (MB) Ecoregion). Aerial photographs: Dave Downing (Timberline Natural Resource Group). Ground photographs and photograph of cloudberry: Bob Decker (Government of the Northwest Territories). Other plant photographs: Christian Bucher. Members of the Ecosystem Classification Group Dave Downing Ecologist, Timberline Natural Resource Group, Edmonton, Alberta. Bob Decker Forest Ecologist, Forest Management Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Hay River, Northwest Territories. Bas Oosenbrug Habitat Conservation Biologist, Wildlife Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, Northwest Territories. Charles Tarnocai Research Scientist, Agriculture and Agri-Food Canada, Ottawa, Ontario. Tom Chowns Environmental Consultant, Powassan, Ontario. Chris Hampel Geographic Information System Specialist/Resource Analyst, Timberline Natural Resource Group, Edmonton, Alberta. -
Arctic Environmental Strategy Summary of Recent Aquatic Ecosystem Studies Northern Water Resources Studies
Arctic Environmental Strategy Summary of Recent Aquatic Ecosystem Studies Northern Water Resources Studies Arctic Environmental Strategy Summary ofRecent Aquatic Ecosystem Studies August 1995 Northern Affairs Program Edited by J. Chouinard D. Milburn Published under the authority of the Honourable Ronald A. Irwin, P.C., M.P., Minister of Indian Affairs and Northern Development Ottawa, 1995 QS-8507-030-EF-Al Catalogue No. R72-244/1-1995E ISBN 0-662-23939-3 © Minister of Public Works and Government Services Canada FOREWORD The Arctic Environmental Strategy (AES), announced in April 1991, is a six-year $100 million Green Plan initiative. The overall goal ofthe AES is to preserve and enhance the integrity, health, biodiversity and productivity ofour Arctic ecosystems for the benefit ofpresent and future generations. Four specific programs address some ofthe key environmental challenges: they are waste cleanup, contaminants, water management, and environment and economy integration. The programs are managed by the Northern Affairs Program ofthe Department of Indian Affairs and Northern Development (DIAND); however, there is a strong emphasis on partnerships with northern stakeholders including Native organizations, other federal departments and the territorial governments. The AES Action on Water Program specifically strives to enhance the protection ofnorthern freshwaters through improved knowledge and decision-making. Water Resources managers in the Yukon and the Northwest Territories administer this Program which focuses on freshwater aquatic ecosystems. This report is the first detailed compilation ofstudies.conducted under the AES Action on Water Program. It covers work done from 1991 to 1994. Many studies have been concluded, while others are ongoing. Although data may not be available for all studies, or results are preliminary at this time, this report presents detailed background, objectives and methodology. -

An Arctic Engineer's Story 1971 to 2006
An Arctic Engineer’s Story 1971 to 2006 by Dan Masterson To my wife Ginny and my sons, Andrew, Greg, and Mark ii Preface In 1971, I just happened to be in the right place at the right time. I had just completed my PhD and was looking for work. I was told to contact Hans Kivisild who had just been given a contract from an oil company to investigate an engineering issue in the Arctic. This started my career in Arctic engineering, just when the second major exploration phase was beginning in the western Arctic, 123 years after Franklin started the first phase of exploration in the area. This recent phase was also filled with individuals who were going “where few had gone before,” but unlike the earlier explorers, these recent explorers were accompanied by regulators, scientists and engineers who wanted to ensure that the environment was protected and also to ensure that the operations were carried out in the safest and most cost- efficient manner. Between about 1970 to 1995, several oil companies and Arctic consulting companies turned Calgary into a world leader in Arctic technology. It was a time that one could have an idea, check it out in small scale, and within a year or so, use it in a full-scale operation. During the next 25 years, industry drilled many wells in the Arctic using the technologies described in this book. In 1995, the oil industry pulled out of the Arctic mainly due to lack of government incentives and poor drilling results. In 2016, both the Canadian and United States governments declared a moratorium on Arctic drilling. -
Project Description of the Trans-Alaska Pipeline System
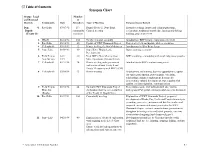
Synopsis Chart Group: Local Number and Regional of Districts Community Date Attendees Type of Meeting Purpose/Issues Raised Dene *1 Rae-Edzo 07/07/92 15+ Dogrib Treaty 11 Dene Band Extensive staking, future land claim negotiations, Dogrib community Council meeting reclamation, traditional knowledge, hunting and fishing, (Treaty 11) members training, project overview 2 Wha Ti 08/24/92 300 Treaty 11 annual assembly Introduction, BHP history, exploration overview 3 Rae-Edzo 05/14/93 40 Update of NWT Diamonds Project Project overview and update; slide presentation 4 Yellowknife 07/15/93 12 Dinner for Dogrib, Gov’t Ministers Introductory, held by Brian Loton 5 Snare Lake 09/09/93 40 Snare River Hydroelectric Dam renaming ceremony Development 6 Field Trip to 12/1- 30 Visit BHP’s New Mexico Coal BHP’s working relationship with local indigenous peoples New Mexico 3/93 Mine Operations (Navajo Nation) 7 Yellowknife 02/21/94 34 Dinner meeting with government Introduction to BHP’s senior management and leaders of both Treaty 8 and Treaty 11 councils with BHP’s CEO 8 Yellowknife 03/08/94 23 Dinner meeting Employment and training, business opportunities, option for equity participation, power supply, education, scholarships, summer employment, heritage site preservation, cultural development, water quality, fish quality, caribou migration, communications 9 Field Trip to 03/10/94 24 To show NWT Diamonds Project Presentation made, visit to Koala drill site, visit to Mine Site exploration work to representatives underground Fox portal, environmental concerns -

Canada Topographical
University of Waikato Library: Map Collection Canada: topographical maps 1: 250,000 The Map Collection of the University of Waikato Library contains a comprehensive collection of maps from around the world with detailed coverage of New Zealand and the Pacific : Editions are first unless stated. These maps are held in storage on Level 1 Please ask a librarian if you would like to use one: Coverage of Canadian Provinces Province Covered by sectors On pages Alberta 72-74 and 82-84 pp. 14, 16 British Columbia 82-83, 92-94, 102-104 and 114 pp. 16-20 Manitoba 52-54 and 62-64 pp. 10, 12 New Brunswick 21 and 22 p. 3 Newfoundland and Labrador 01-02, 11, 13-14 and 23-25) pp. 1-4 Northwest Territories 65-66, 75-79, 85-89, 95-99 and 105-107) pp. 12-21 Nova Scotia 11 and 20-210) pp. 2-3 Nunavut 15-16, 25-27, 29, 35-39, 45-49, 55-59, 65-69, 76-79, pp. 3-7, 9-13, 86-87, 120, 340 and 560 15, 21 Ontario 30-32, 40-44 and 52-54 pp. 5, 6, 8-10 Prince Edward Island 11 and 21 p. 2 Quebec 11-14, 21-25 and 31-35 pp. 2-7 Saskatchewan 62-63 and 72-74 pp. 12, 14 Yukon 95,105-106 and 115-117 pp. 18, 20-21 The sector numbers begin in the southeast of Canada: They proceed west and north. 001 Newfoundland 001K Trepassey 3rd ed. 1989 001L St: Lawrence 4th ed. 1989 001M Belleoram 3rd ed. -

High Arctic PEMT Layers
High Arctic PEMT Layers Table of Contents General Sensitivity Notes .............................................................................................................................. 3 Sensitivity Layers ........................................................................................................................................... 3 Grid Cell Sensitivity Rating ............................................................................................................................ 3 Polar Bear ...................................................................................................................................................... 3 Rationale for Selection .............................................................................................................................. 3 Key habitat ................................................................................................................................................ 4 Sustainability Factors ................................................................................................................................ 4 Susceptibility to Oil and Gas Activities ...................................................................................................... 5 Potential Effects of Climate Change ......................................................................................................... 5 Sensitivity Ranking ................................................................................................................................... -

Kivalliq Musk Ox Management Plan
Page 1 of 20 DRAFT MANAGEMENT PLAN FOR HIGH ARCTIC MUSKOXEN OF THE QIKIQTAALUK REGION 2012 – 2017 Prepared in collaboration with: NTI Wildlife, Resolute Bay HTO, Arctic Bay HTO, Grise Fiord HTO and the Qikiqtaaluk Wildlife Board Draft Management Plan for High Arctic Muskoxen of the Qikiqtaaluk Region Page 2 of 20 TABLE OF CONTENTS 1.0 SUMMARY 2.0 THE QIKIQTAALUK MUSKOX POPULATION AND RANGE 2.1 Muskox Range 2.2 Communities that Harvest Muskoxen 3.0 THE NEED FOR A MUSKOX MANAGEMENT PLAN 4.0 ROLES OF CO-MANAGEMENT PARTNERS AND STAKEHOLDERS 4.1 Role of the Co-Management Partners 4.2 Role of the Communities 4.3 Inclusion of Inuit Qaujimajatuqangit 5.0 QIQIKTAALUK MUSKOX MANAGEMENT PLAN 5.1 Goals of the Management Plan 5.2 Management Plan Priorities 5.3 Management Strategies 5.4 Herd Monitoring and Indicators 5.5 Muskox Management Units 6.0 Action Plans Appendix A - RECOMMENDATIONS Appendix B – ACTION PLAN Draft Management Plan for High Arctic Muskoxen of the Qikiqtaaluk Region Page 3 of 20 1.0 SUMMARY Prior to the enactment of protection in 1917 (Burch, 1977), muskox populations throughout the central Arctic were hunted to near extinction. Although limited information is available on the status of muskoxen in much of the eastern Mainland (Fournier and Gunn, 1998), populations throughout Nunavut are currently re-colonizing much of their historical range. Qikiqtaaluk harvesters have reported increased sightings of muskoxen in close proximity to their communities which indicates that the animals have expanded their ranges significantly over the last few decades. The Qikiqtaaluk Muskox Management Plan will serve as a tool to assist the Nunavut Wildlife Management Board (NWMB), the Qikiqtaaluk Wildlife Board (QWB), Government of Nunavut Department of Environment (DoE) and Nunavut Tunngavik Inc. -

About the Aurora Research Institute
This publication is a collaboration between the Aurora Research Institute, The Canadian Department of Fisheries and Oceans, Department of Environment and Natural Resources, Government of the Northwest Territories and The Prince of Wales Northern Heritage Centre. Thank you to all who submitted a summary of their research, photographs and helped make this publication possible. Editor: Karen Heikkila, Aurora Research Institute Andrew Applejohn, Aurora Research Institute Copyright © 2008 ISSN: 1205-3910 Printed in Fort Smith through the Aurora Research Institute ABOUT THE AURORA RESEARCH INSTITUTE The Aurora Research Institute (ARI) was established in 1995 as a division of Aurora College when the Science Institute of the Northwest Territories (NWT) divided into eastern (Nunavut) and western (NWT) divisions. The Aurora Research Institute’s mandate is to improve the quality of life for NWT residents by applying scientific, technological and Indigenous Knowledge to solve northern problems and advance social and economic goals. ARI is responsible for: • licensing and coordinating research in accordance with the NWT Scientists Act: This covers all disciplines including the physical, social, biological sciences and Traditional Knowledge; • promoting communication between researchers and the people of the communities in which they work; • promoting public awareness of the importance of science, technology and Indigenous Knowledge; • fostering a scientific community within the NWT which recognizes and uses the Traditional Knowledge of northern aboriginal people; • making scientific and Indigenous Knowledge available to the people of the NWT; • supporting or conducting research and technological developments which contribute to the social, cultural and economic prosperity of the people of the NWT To learn more about ARI, contact us at: Aurora Research Institute 191 Mackenzie Road P.O. -

North Magnetic Dip Pole (NMDP)
Journey to the Top (or the Relocated Dip) Ray Talson, Search & Rescue Society of BC The North Magnetic Pole - of interest to navigators for centuries - but no one knew exactly where it was. A study was done by the Department of Energy, Mines and Resources, Geological survey of Canada, Geophysics Division, to determine the exact location of the North Magnetic Dip Pole (NMDP). This is the location where the north seeking end of the compass needle wants to point straight into the ground. The study was published by the Canadian Journal of Earth Sciences, Vol. 23, 1986. The study was authored by L.R. Newitt and E.R. Niblett. This review is based on pages 1062 through 1067 of that study. Back in 1831 a man by the name of J.C.ROSS (Ross 1834) was the first person to plot where the NORTH MAGNETIC POLE was. Its rapid motion, 800 km in 150 years (Barraclough and Malin 1981), has led to the re-determination of its position several times since. It has been customary for the Earth Physics Branch, Department of Energy, Mines, and Resources, Canada, to re- determine its position approximately once every decade. The pole has moved approximately 750 km since 1904, an average of 9.4 km per year. From 1973, when the pole position was last determined, to late 1983, the movement has been approximately 120 km, an average of 11.6 km per year, only slightly more than the 80 year average. Its present direction of travel is approximately northwest. The North Magnetic Pole was found to be very active and elusive. -

Queen Elizabeth Islands Game Survey, 1961
•^i.ri.iri^VJU.HIJJ^^^iiH^ Queen Elizabeth Islands Game Survey, 1961 by JOHN S. TENER OCCASIONAL PAPERS No. 4 QUEEN ELIZABETH ISLANDS GAME SURVEY, 1961 by John S. Tener Canadian Wildlife Service Occasional Papers Number 4 National Parks Branch Department of Northern Affairs and National Resources Issued under the authority of the HONOURABLE ARTHUR LAING, P.C, M.P., B.S.A.. Minister of Northern Affairs and National Resources ROGER DUHAMEL, F.R.S.C. Queen's Printer and Controller oi Stationery, Ottawa, 1963 Cat. No. R 69-1/4 CONTENTS 5 ABSTRACT 6 INTRODUCTION 7 OBJECTIVE Personnel, 7 Itinerary, 7 8 METHOD Logistics, 8 Survey design, 8 Survey method, 9 Sources of error, 11 13 RESULTS Caribou and muskoxen on the Islands Devon, 14 Mackenzie King, 28 Cornwallis, 14 Brock, 29 Little Cornwallis, 15 Ellef Ringnes, 29 Bathurst, 15 Amund Ringnes, 30 Melville, 20 Lougheed, 31 Prince Patrick, 23 King Christian, 32 Eglinton, 26 Cornwall, 32 Emerald, 27 Axel Heiberg, 32 Borden, 27 Ellesmere, 33 38 SPECIES SUMMARY Avifauna American and Gyrfalcon, 40 black brant, 38 Peregrine falcon, 41 Snow geese, 39 Rock ptarmigan, 41 Old Squaw, 40 Snowy owl, 41 King eider, 40 Raven, 41 Mammalian fauna Polar bear, 42 Seals, 44 Arctic fox, 43 Peary caribou, 45 Arctic wolf, 44 Muskoxen, 47 49 SUMMARY 50 REFERENCES LIST OF TABLES I Survey intensity of the Islands, 13 II Calculations, estimated caribou population, Bathurst Island, 18 III Calculations, estimated muskox population, Bathurst Island, 19 IV Observations from the ground of four muskox herds, Bracebridge Inlet, -

Wildlife Research in the NWT
2013 Annual Report of 2012 Wildlife Research In The NWT Photo: GNWT/A. D’Hont, ENR Photo: GNWT/J. Adamczewski, ENR ISBN: 978-0-7708-0214-1 Contents ENR Administrative Regions of the NWT................................. 5 Map of the Northwest Territories........................................ 6 Introduction............................................................ 7 Wildlife Species Research BATS Bat Monitoring in the Northwest Territories . 8 BEARS Inuvik-Tuktoyaktuk Highway Grizzly Bear Denning Survey . 12 Grizzly Bear Denning Survey for the Inuvik - Tuktoyaktuk Highway . 16 Reducing Human-Bear Conflicts on the Dempster Highway . 18 Viscount Melville Sound Polar Bear Subpopulation Survey . 20 Joint Regional Grizzly Bear DNA Hair Snagging Program . 24 Black Bear Ecology in the North Slave Region. 28 BEVERLY AND AHIAK CARIBOU Distribution and Movements of Beverly and Ahiak Barren-ground Caribou . 32 BIRDS Bioelectronic Monitoring of Peregrine Falcons along the Mackenzie River, NWT . 34 Cooperative Waterfowl Population Surveys in the Northwest Territories . 36 Snow Goose Population Study in the Inuvialuit Settlement Region . 40 Breeding Bird Surveys in the Gwich’in Settlement Area, Routes 1 to 4 . 42 Survey of Seabird Colony at Cape Parry Migratory Bird Sanctuary . 44 Arctic Shorebird Monitoring Program . 46 Monitoring Oil Sands Contaminants in Migrating Waterfowl . 50 Densities and Population Trends of Tundra Birds at TERS Daring Lake, NWT . 52 Gull Surveys on Frame Lake, Yellowknife . 54 1 Vocalizations and Other Sounds as Tools to Document Population Structure of the Wilson’s Snipe Across its North American Breeding Range . 58 Western Canada Cooperative Banding Program – Stagg River Station . 60 Whooping Crane Ecology and Rehabilitation . 62 Western Canada Cooperative Pre-season Waterfowl Banding Program – Mills Lake Station .