Guidelines for the Appropriate Use of Blood and Blood Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Transfusions
Guidelines for Transfu- sion Prepared by: Community Transfusion Committee Lincoln, Nebraska CHAIR: Aina Silenieks, MD MEMBERS: L. Bausch, M.D. R. Burton, M.D. S. Dunder, M.D. D. Voigt, M.D. B. J. Wilson, M.D. COMMUNITY Becky Croner REPRESENTATIVES: Ellen DiSalvo Christa Engel Phyllis Ericson Kelly Gillaspie Pat Gilles Vic Grdina Jessica Henrichs Kelly Jensen Laurel McReynolds Christina Nickel Angela Novotny Kelley Thiemann Janet Wachter Jodi Wikoff Guidelines For Transfusion Community Transfusion Committee INTRODUCTION The Community Transfusion Committee is a multidisciplinary group that meets to monitor blood utilization practices, establish guidelines for transfusion and discuss relevant transfusion related topics. It is comprised of physicians from local hospitals, invited guests, and community representatives from the hospitals’ transfusion services, nursing services, perfusion services, health information management, and the Nebraska Community Blood Bank. These Guidelines for Transfusion are reviewed and revised biannually by the Community Trans- fusion Committee to ensure that the industry’s most current practices are promoted. The Guidelines are the standard by which utilization practices are evaluated. They are also de- signed to provide helpful information to assist physicians to provide appropriate blood compo- nent therapy to patients. Appendices have been added for informational purposes and are not to be used as guidance for clinical decision making. ADULT RED CELLS A. Indications 1. One of the following a. Hypovolemia and hypoxia (signs/symptoms: syncope, dyspnea, postural hypoten- sion, tachycardia, angina, or TIA) secondary to surgery, trauma, GI tract bleeding, or intravascular hemolysis, OR b. Evidence of acute loss of 15% of total blood volume or >750 mL blood loss, OR c. -

27. Clinical Indications for Cryoprecipitate And
27. CLINICAL INDICATIONS FOR CRYOPRECIPITATE AND FIBRINOGEN CONCENTRATE Cryoprecipitate is indicated in the treatment of fibrinogen deficiency or dysfibrinogenaemia.1 Fibrinogen concentrate is licenced for the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency, including afibrinogenaemia and hypofibrinogenaemia,2 and is currently funded under the National Blood Agreement. Key messages y Fibrinogen is an essential component of the coagulation system, due to its role in initial platelet aggregation and formation of a stable fibrin clot.3 y The decision to transfuse cryoprecipitate or fibrinogen concentrate to an individual patient should take into account the relative risks and benefits.3 y The routine use of cryoprecipitate or fibrinogen concentrate is not advised in medical or critically ill patients.2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In the setting of major obstetric haemorrhage, early administration of cryoprecipitate or fibrinogen concentrate may be necessary.3 Clinical implications y The routine use of cryoprecipitate or fibrinogen concentrate in medical or critically ill patients with coagulopathy is not advised. The underlying causes of coagulopathy should be identified; where transfusion is considered necessary, the risks and benefits should be considered for each patient. Specialist opinion is advised for the management of disseminated intravascular coagulopathy (MED-PP18, CC-PP7).2,4 y Cryoprecipitate or fibrinogen concentrate may be indicated in critical bleeding if fibrinogen levels are not maintained using FFP. In patients with critical bleeding requiring massive transfusion, suggested doses of blood components is 3-4g (CBMT-PP10)3 in adults or as per the local Massive Transfusion Protocol. -

Blood Product Replacement: Obstetric Hemorrhage
CMQCC OBSTETRIC HEMORRHAGE TOOLKIT Version 2.0 3/24/15 BLOOD PRODUCT REPLACEMENT: OBSTETRIC HEMORRHAGE Richard Lee, MD, Los Angeles County and University of Southern California Medical Center Laurence Shields, MD, Marian Regional Medical Center/Dignity Health Holli Mason, MD, Cedars-Sinai Medical Center Mark Rollins, MD, PhD, University of California, San Francisco Jed Gorlin, MD, Innovative Blood Resources/Memorial Blood Center, St. Paul, Minnesota Maurice Druzin, MD, Lucile Packard Children’s Hospital Stanford University Jennifer McNulty, MD, Long Beach Memorial Medical Center EXECUTIVE SUMMARY • Outcomes are improved with early and aggressive intervention. • Both emergency blood release and massive transfusion protocols should be in place. • In the setting of significant obstetric hemorrhage, resuscitation transfusion should be based on vital signs and blood loss and should not be delayed by waiting for laboratory results. • Calcium replacement will often be necessary with massive transfusion due to the citrate used for anticoagulation in blood products. • During massive transfusion resuscitation, the patient’s arterial blood gas, electrolytes, and core temperature should be monitored to guide clinical management and all transfused fluids should be warmed; direct warming of the patient should be initiated as needed to maintain euthermia and to avoid added coagulopathy. BACKGROUND AND LITERATURE REVIEW After the first several units of packed red blood cells (PRBCs) and in the face of continuing or worsening hemorrhage, aggressive transfusion therapy becomes critical. This report covers the experience with massive transfusion protocols. Lessons from military trauma units as well as civilian experience with motor vehicle accidents and massive obstetric hemorrhage have identified new principles such as earlier use of plasma (FFP/thawed plasma/plasma frozen within 24 hours/liquid plasma) and resuscitation transfusion while laboratory results are pending. -

Platelet Transfusion
Lab Dept: Transfusion Services Test Name: PLATELET TRANSFUSION General Information Lab Order Codes: TPLT Special charges: HLA Matched-MHLA, AHLA PLA1 Negative – PLAT, APLA1 Platelet Crossmatching PLTX – CPLT Synonyms: Leukocyte Reduced Platelets; Random Platelets; Platelet Rich Plasma; Platelet concentrate; Leukocyte Reduced Platelet Pheresis; Single- Donor Platelets; SDP Platelet pheresis; Apheresis Platelets; LRPH CPT Codes: P9035 – Platelet pheresis, Leukocyte reduced 86806 – Lymphocytotoxicity assay, visual crossmatch, without titration 86813 – HLA Matched 86945 – Irradiation 86985 – Volume Reduction 86965 – Pooling 86903 – Platelet Antigen typing 86999 – Washing 86022 – Platelet Crossmatch Test Includes: Leukocyte Reduced Platelet Pheresis consists of platelets suspended in 200-300 mL of plasma collected by cytapheresis. Each unit contains at least 3 x 1011 platelets, and ≤5.0 x 106 leukocytes. One pheresis unit equals 5-6 random unit platelet concentrate. Logistics Test Indications: Refer to Guidelines for the Transfusion of Blood Components. Platelet Crossmatching or HLA-Matched Platelets may be useful for patients receiving repeated platelet transfusions who have become refractile, and for patients who repeatedly develop febrile non-hemolytic transfusion reactions after platelet transfusions. Volume Reduced Platelets are indicated in the event that ABO - incompatible platelets must be transfused due to availability or in patients with severe fluid restrictions. Refer to Blood Component Compatibility Chart Lab Testing Sections: Transfusion Services Phone Numbers: MIN Lab: 612-813-6824 STP Lab: 651-220-6558 Test Availability: Daily, 24 hours Turnaround Time: 1 - 2 hours Standard Dose/Volume: <10 kg: 10 – 15 mL/kg up to one unit or 50 mL maximum 10 – 15 kg: 1/3 pheresis unit 15 – 25 kg: 1/2 pheresis unit >25 kg: 1 pheresis unit Rate of Infusion: 10 minutes/unit, <4 hours Administration: Must be administered through a blood component administration filter. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Red Blood Cells, Leukocyte Reduced Covenant and AHS Sites
This document applies to all Red Blood Cells, Leukocyte Reduced Covenant and AHS sites. Class: Human blood component, derived from OTHER NAMES: Packed red blood cells, Packed whole blood cells INTRAVENOUS OTHER ROUTES DIRECT IV Intermittent Continuous Infusion Infusion SC IM OTHER Acceptable No Yes No No No No Routes* * Professionals performing these restricted activities have received authorization from their regulatory college and have the knowledge and skill to perform the skill competently. DESCRIPTION OF PRODUCT: Red Blood Cell (RBC) concentrates are prepared from approximately 480mL whole blood usually collected in 70mL of anticoagulant. The unit is plasma reduced by centrifugation, platelet reduced by either centrifugation or filtration and leukocyte reduced by filtration. RBCs are typically resuspended in approximately 110mL of nutrient. Collected from volunteer donors by the Canadian Blood Services (CBS). Donor is screened and blood is tested for: i. Hepatitis B Surface Antigen (HBsAg) ii. Syphilis iii. Antibodies to Hepatitis B core antigen (HBcore), Hepatitis C Virus (HCV), Human T-cell Lymphotropic Virus (HTLV-1 and 2), Human Immune Deficiency Virus (HIV-1 and 2) iv. Nucleic Acid Testing (NAT) for presence of HCV RNA, HIV-1 RNA and West Nile Virus (WNV) RNA . CBS also tests blood for ABO/Rh and clinically significant antibodies. Some donors are tested for CMV status. RBCs do NOT contain any coagulation factors or platelets. AVAILABILITY: . Contact your local transfusion service/laboratory for ABO and Rh types stocked at your site. ABO compatible (not always identical) may be required if the transfusion service cannot provide sufficient quantities of the patient’s blood group (See ABO compatibility chart at: http://www.albertahealthservices.ca/assets/wf/lab/wf-lab-clin-tm-abo-compatability.pdf). -

Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/Jp-Journals-10071-23250
INVITED ARTICLE Transfusion Triggers for Platelets and Other Blood Products Sunil Karanth Indian Journal of Critical Care Medicine (2019): 10.5005/jp-journals-10071-23250 INTRODUCTION Department of Intensive Care Unit, Manipal Hospital, Bengaluru, Transfusion of whole blood is not associated with any significant Karnataka, India benefit, rather can be harmful. Significant advances have been Corresponding Author: Sunil Karanth, Department of Intensive made in transfusion medicine, facilitating the use of blood product Care Unit, Manipal Hospital, Bengaluru, Karnataka, India, e-mail: or component therapy than use of whole blood (Fig. 1). In 2009, a [email protected] report on Serious Hazards of Transfusion in the UK, estimated that How to cite this article: Karanth S. Transfusion Triggers for Platelets a total of 3 million units of blood components were released. The and Other Blood Products. Indian J Crit Care Med 2019;23(Suppl requirement of the same in South-East Asia is much higher to the 3):S189–S190. tune of 15 million units annually. The current recommendation Source of support: Nil is to use blood only in life-threatening situations, rather than to Conflict of interest: None normalize abnormal numbers. PLATELETS Platelets are derived from the buffy coat of whole blood donations. A pooled platelet concentrate includes pooled buffy-coat derived platelets from four whole-blood donations suspended in platelet additive solution and plasma of one of the four donors. It contains 240000 platelets pooled from 4–6 donors. A Single donor platelet is derived from a single-donor by a process of apheresis. In view of the lesser number of donors and the theoretical advantage of involving a single-donor platelet (SDP) may be preferred over the use of platelets from multiple donors. -

Blood-Platelet-Orders-Outpatient-V3
Blood / Platelet Orders Outpt v3 USE THIS ORDER SET FOR OUTPATIENT NON URGENT BLOOD OR PLATELET TRANSFUSION 24 Hours advanced notice required for infusion center NO MORE THAN 2 UNITS OF RED BLOOD CELLS CAN BE INFUSED IN THE INFUSION CENTER PER DAY BMH Outpatient Infusion Center: Fax completed order set to 843-522-7313/Phone 843-522-7680 Hospital Outpatient: Fax Completed order set to Registration at 843-522-5741 and notify Nursing Supervisor at 843-522-7653 Service Designation THIS IS NOT AN ADMISSION SET Patient Name ___________________________________ Patient DOB ____________ Service Designation Blood Platelet Outpatient Attending ___________________________________ Date Requested ___________________________________ Status Outpatient Check Appropriate Diagnosis Below: [ Anemia of chronic renal disease Anemia related to chemotherapy Aplastic anemia Anemia related to cancer Anemia related to blood loss Sickle cell disease Anemia unspecified Thombocytopenia (platelets) Other _________________________ ] Allergies Update Allergies in the Summary Panel in MEDITECH Special Requirement: (REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE) Special Requirement RBC or Platelets REQUIRES SPECIAL ORDER ONE DAY IN ADVANCE [ Irradiated CMV Negative HgBS Negative ] Medications diphenhydrAMINE HCl (Benadryl) 25 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 50 mg orally single dose premedicate prior to transfusion diphenhydrAMINE HCl (Benadryl) 25 mg intravenously single dose premedicate prior to transfusion diphenhydrAMINE -

FDA Regulation of Blood and Blood Components in the United States
FDA Regulation of Blood and Blood Components in the United States SLIDE 1 This presentation will review the FDA Regulation of Blood and Blood Components in the U.S. SLIDE 2 The FDA Center for Biologics Evaluation and Research, or CBER, Office of Blood Research and Review, called OBRR, reviews several different types of regulatory applications with respect to blood and blood components. This includes biologics license applications, called BLAs, which represent the regulatory pathway for blood components. The BLA regulatory process also applies to biological drugs such as fractionated plasma products. OBRR also regulates in-vitro diagnostic devices used for screening of collected blood, and does so also using the BLA regulatory pathway. Review of these devices as biologic licenses allows CBER to apply a higher level of manufacturing oversight, including lot release testing and pre-licensure inspection. This regulatory pathway applies to infectious disease tests for blood screening, as well as blood grouping and phenotyping reagents. SLIDE 3 The regulatory pathway for New Drug Applications, or NDAs, is most commonly used within the Center for Drug Evaluation and Research, or CDER. Within the Office of Blood, several NDA applications are reviewed each year, mostly involving solutions used for the collection of blood, such as anticoagulants and red cell nutritive solutions. Interestingly, a blood bag that does not have a solution inside is regulated as a device. If the bag has a solution, then it is a drug-device combination product, but is regulated as a drug. SLIDE 4 OBRR reviews both Class Two and Class Three devices. Class Three devices in OBRR include diagnostic tests for HIV regulated as pre-market approvals, called PMAs. -
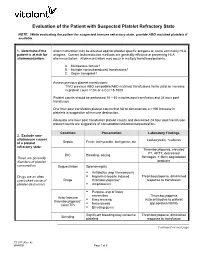
Platelet Transfusion Refractoriness
Evaluation of the Patient with Suspected Platelet Refractory State NOTE: While evaluating the patient for suspected immune refractory state, provide ABO matched platelets if available. 1. Determine if the Alloimmunization may be directed against platelet specific antigens or, more commonly HLA patient is at risk for antigens. Current leukoreduction methods are generally effective at preventing HLA alloimmunization: alloimmunization. Alloimmunization may occur in multiply transfused patients. A. Multiparous female? B. Multiple nonleukoreduced transfusions? C. Organ transplant? Assess previous platelet transfusions: TWO previous ABO compatible/ABO matched transfusions fail to yield an increase in platelet count >10K or a CCI >5-7500 Platelet counts should be performed 10 – 60 minutes post transfusion and 24 hour post transfusion. One hour post transfusion platelet counts that fail to demonstrate a >10K increase in platelets is suggestive of immune destruction. Adequate one hour post transfusion platelet counts and decreased 24 hour post transfusion platelet counts are suggestive of consumption/utilization/sequestration. Condition Presentation Laboratory Findings 2. Exclude non- alloimmune causes Leukocytosis, +cultures Sepsis Fever, tachycardia, tachypnea, etc. of a platelet refractory state: Thrombocytopenia, elevated PT, APTT, decreased DIC Bleeding, oozing These are generally fibrinogen, + fibrin degradation disorders of platelet products consumption. Sequestration Splenomegaly . Antibiotics (esp Vancomycin) Drugs are an often . Heparin (Heparin induced Thrombocytopenia, diminished overlooked cause of Drugs thrombocytopenia)* response to transfusion platelet destruction. Amphotericin . Purpura, esp of lower extremities Thrombocytopenia Auto-Immune . Easy bruising Auto antibodies to platelet thrombocytopenia* Nose bleeds glycoprotein IIb/IIIa (aka ITP) . Bleeding gums Significant bleeding may consume Thrombocytopenia, diminished Bleeding platelets response to transfusion Continued on next page TS 017 (Rev. -

Comparing Platelet Compatibility to Red Cell Compatibility Protocols
Review: comparing platelet compatibility to red cell compatibility protocols S. ROLIH Between 1971 and 1980, the number of platelet con- Incidence of Alloimmunization From centrates transfused in the United States increased from Transfusion 0.4 to 2.8 million units.1 That number increased to near- Each RBC or platelet transfusion has the potential to ly 5 million in 1987 and continues to increase in the influence the outcome of future transfusions of the 1990s. The increase in platelet transfusions has led to a same products by inducing the formation of alloanti- concomitant rise in the incidence of platelet alloimmu- bodies. For RBC transfusions, in which ABO and D nization. As a consequence, many transfusion services group-compatible units are routinely given, this is not a or blood product providers have developed platelet significant problem. Only 0.3 percent to 1.3 percent of compatibility protocols to select platelet components all RBC transfusion recipients produce alloantibodies, that will have acceptable survival when transfused to although in selected recipient populations, such as alloimmunized patients. Most serologists are familiar patients with thalassemia or sickle cell anemia, the with compatibility tests used to select red blood cells alloimmunization rate may be as high as 30 percent (RBCs) for transfusion. These tests are performed before because of chronic transfusions.2 Overall, decreased sur- transfusion, whether or not alloimmunization has vival of transfused RBCs due to alloimmunization occurs occurred. Platelet compatibility tests, in contrast, are infrequently. Once alloimmunization occurs, premature implemented only after alloimmunization and develop- loss of future transfused RBCs is prevented by the selec- ment of the refractory state. -

Red Blood Cell Transfusions –
Peer Reviewed VETcpd - Internal Medicine Jenny Walton BVM&S MRCVS Jenny qualified from R(D)SVS in Red blood cell transfusions – 1998 - she worked in mixed practice when, what and how to do it! for 4 years before moving into the Red cell transfusions are now a relatively common intervention in veterinary practice field of small in the UK and help in the treatment of many patients. This is largely due to the animal emergency availability of blood products, such as Packed Red Blood Cells (PRBC) , from blood and critical care banks supplying directly to practices. After donation, red cells are separated from with Vets Now for 12 years. Through plasma into a concentrated packed cell form, a nutrient extender is then added to Vets Now, she ran the practical trial them. This allows the red cells to be stored for up to 42 days before being transfused researching canine blood banking in 2005-2006 – launching Pet Blood Bank into patients. DEA 1 blood typing prior to transfusion is essential and cross matching UK (PBB) alongside Wendy Barnett should be performed for second transfusions. Blood products are administered in 2007. She acts as the Veterinary through a filtered giving set and patients monitored closely for transfusion reactions. Supervisor for PBB, her role includes Transfusion reactions are thankfully rare but potentially life threatening. advising practitioners daily on the appropriate use of PBB blood products, Key words: Canine, Packed Red Blood Cells (PRBC), transfusion, blood typing, overseeing the practical and VMD cross matching, transfusion reactions legislative veterinary aspects of blood collection at PBB and leading research on future development opportunities.