Allotropes of Carbon It‟S All in the Way You‟Re Put Together
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
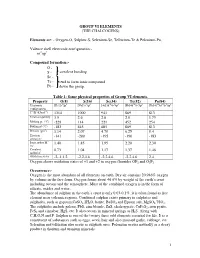
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

Graphite Materials for the U.S
ANRIC your success is our goal SUBSECTION HH, Subpart A Timothy Burchell CNSC Contract No: 87055-17-0380 R688.1 Technical Seminar on Application of ASME Section III to New Materials for High Temperature Reactors Delivered March 27-28, 2018, Ottawa, Canada TIM BURCHELL BIOGRAPHICAL INFORMATION Dr. Tim Burchell is Distinguished R&D staff member and Team Lead for Nuclear Graphite in the Nuclear Material Science and Technology Group within the Materials Science and Technology Division of the Oak Ridge National Laboratory (ORNL). He is engaged in the development and characterization of carbon and graphite materials for the U.S. Department of Energy. He was the Carbon Materials Technology (CMT) Group Leader and was manager of the Modular High Temperature Gas-Cooled Reactor Graphite Program responsible for the research project to acquire reactor graphite property design data. Currently, Dr. Burchell is the leader of the Next Generation Nuclear Plant graphite development tasks at ORNL. His current research interests include: fracture behavior and modeling of nuclear-grade graphite; the effects of neutron damage on the structure and properties of fission and fusion reactor relevant carbon materials, including isotropic and near isotropic graphite and carbon-carbon composites; radiation creep of graphites, the thermal physical properties of carbon materials. As a Research Officer at Berkeley Nuclear Laboratories in the U.K. he monitored the condition of graphite moderators in gas-cooled power reactors. He is a Battelle Distinguished Inventor; received the Hsun Lee Lecture Award from the Chinese Academy of Science’s Institute of Metals Research in 2006 and the ASTM D02 Committee Eagle your success is our goal Award in 2015. -

The Era of Carbon Allotropes Andreas Hirsch
commentary The era of carbon allotropes Andreas Hirsch Twenty-five years on from the discovery of 60C , the outstanding properties and potential applications of the synthetic carbon allotropes — fullerenes, nanotubes and graphene — overwhelmingly illustrate their unique scientific and technological importance. arbon is the element in the periodic consist of extended networks of sp3- and 1985, with the advent of fullerenes (Fig. 1), table that provides the basis for life sp2 -hybridized carbon atoms, respectively. which were observed for the first time by Con Earth. It is also important for Both forms show unique physical properties Kroto et al.3. This serendipitous discovery many technological applications, ranging such as hardness, thermal conductivity, marked the beginning of an era of synthetic from drugs to synthetic materials. This lubrication behaviour or electrical carbon allotropes. Now, as we celebrate role is a consequence of carbon’s ability conductivity. Conceptually, many other buckminsterfullerene’s 25th birthday, it is to bind to itself and to nearly all elements ways to construct carbon allotropes are also the time to reflect on a growing family in almost limitless variety. The resulting possible by altering the periodic binding of synthetic carbon allotropes, which structural diversity of organic compounds motif in networks consisting of sp3-, sp2- includes the synthesis of carbon nanotubes and molecules is accompanied by a broad and sp-hybridized carbon atoms1,2. As a in 19914 and the rediscovery of graphene range of -

Chemistry with Graphene and Graphene Oxide - Challenges for Synthetic Chemists Siegfried Eigler* and Andreas Hirsch*
Chemistry with Graphene and Graphene Oxide - Challenges for Synthetic Chemists Siegfried Eigler* and Andreas Hirsch* Dr. Siegfried Eigler*, Prof. Dr. Andreas Hirsch* Department of Chemistry and Pharmacy and Institute of Advanced Materials and Processes (ZMP) Henkestrasse 42, 91054 Erlangen and Dr.-Mack Strasse 81, 90762 Fürth (Germany) Fax: (+49) 911-6507865015 E-mail: [email protected]; [email protected] The chemical production of graphene as well as its controlled wet- chemical modification is a challenge for synthetic chemists and the characterization of reaction products requires sophisticated analytic methods. In this review we first describe the structure of graphene and graphene oxide. We then outline the most important synthetic methods which are used for the production of these carbon based nanomaterials. We summarize the state-of-the-art for their chemical functionalization by non-covalent and covalent approaches. We put special emphasis on the differentiation of the terms graphite, graphene, graphite oxide and graphene oxide. An improved fundamental knowledge about the structure and the chemical properties of graphene and graphene oxide is an important prerequisite for the development of practical applications. 1. Introduction: Graphene and Graphene Oxide – Opportunities and Challenges for Synthetic Chemists Research into graphene and graphene oxide (GO) represents an emerging field of interdisciplinary science spanning a variety of disciplines including chemistry, physics, materials science, device fabrication and -

CNT Technical Interchange Meeting
Realizing the Promise of Carbon Nanotubes National Science and Technology Council, Committee on Technology Challenges, Oppor tunities, and the Path w a y to Subcommittee on Nanoscale Science, Engineering, and Technology Commer cializa tion Technical Interchange Proceedings September 15, 2014 National Nanotechnology Coordination Office 4201 Wilson Blvd. Stafford II, Rm. 405 Arlington, VA 22230 703-292-8626 [email protected] www.nano.gov Applications Commercial Product Characterization Synthesis and Processing Modeling About the National Nanotechnology Initiative The National Nanotechnology Initiative (NNI) is a U.S. Government research and development (R&D) initiative involving 20 Federal departments, independent agencies, and independent commissions working together toward the shared and challenging vision of a future in which the ability to understand and control matter at the nanoscale leads to a revolution in technology and industry that benefits society. The combined, coordinated efforts of these agencies have accelerated discovery, development, and deployment of nanotechnology to benefit agency missions in service of the broader national interest. About the Nanoscale Science, Engineering, and Technology Subcommittee The Nanoscale Science, Engineering, and Technology (NSET) Subcommittee is the interagency body responsible for coordinating, planning, implementing, and reviewing the NNI. NSET is a subcommittee of the Committee on Technology (CoT) of the National Science and Technology Council (NSTC), which is one of the principal means by which the President coordinates science and technology policies across the Federal Government. The National Nanotechnology Coordination Office (NNCO) provides technical and administrative support to the NSET Subcommittee and supports the Subcommittee in the preparation of multiagency planning, budget, and assessment documents, including this report. -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Carbon Nanotube Research Developments in Terms of Published Papers and Patents, Synthesis and Production
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Scientia Iranica F (2012) 19 (6), 2012–2022 Sharif University of Technology Scientia Iranica Transactions F: Nanotechnology www.sciencedirect.com Carbon nanotube research developments in terms of published papers and patents, synthesis and production H. Golnabi ∗ Institute of Water and Energy, Sharif University of Technology, Tehran, P.O. Box 11555-8639, Iran Received 16 April 2012; revised 12 May 2012; accepted 10 October 2012 KEYWORDS Abstract Progress of carbon nanotube (CNT) research and development in terms of published papers and Published references; patents is reported. Developments concerning CNT structures, synthesis, and major parameters, in terms Paper; of the published documents are surveyed. Publication growth of CNTs and related fields are analyzed for Patent; the period of 2000–2010. From the explored search term, ``carbon nanotubes'', the total number of papers Nanotechnology; containing the CNT concept is 52,224, and for patents is 5,746, with a patent/paper ratio of 0.11. For CNT Synthesis. research in the given period, an annual increase of 8.09% for paper and 8.68% for patents are resulted. Pub- lished papers for CNT, CVD and CCVD synthesis parameters for the period of 2000–2010 are compared. In other research, publications for CNT laser synthesis, for the period of 2000–2010, are reviewed. Publica- tions for major laser parameters in CNT synthesis for the period of 2000–2010 are described. The role of language of the published references for CNT research for the period of 2000–2010 is also investigated. -

Coal Characteristics
CCTR Indiana Center for Coal Technology Research COAL CHARACTERISTICS CCTR Basic Facts File # 8 Brian H. Bowen, Marty W. Irwin The Energy Center at Discovery Park Purdue University CCTR, Potter Center, 500 Central Drive West Lafayette, IN 47907-2022 http://www.purdue.edu/dp/energy/CCTR/ Email: [email protected] October 2008 1 Indiana Center for Coal Technology Research CCTR COAL FORMATION As geological processes apply pressure to peat over time, it is transformed successively into different types of coal Source: Kentucky Geological Survey http://images.google.com/imgres?imgurl=http://www.uky.edu/KGS/coal/images/peatcoal.gif&imgrefurl=http://www.uky.edu/KGS/coal/coalform.htm&h=354&w=579&sz= 20&hl=en&start=5&um=1&tbnid=NavOy9_5HD07pM:&tbnh=82&tbnw=134&prev=/images%3Fq%3Dcoal%2Bphotos%26svnum%3D10%26um%3D1%26hl%3Den%26sa%3DX 2 Indiana Center for Coal Technology Research CCTR COAL ANALYSIS Elemental analysis of coal gives empirical formulas such as: C137H97O9NS for Bituminous Coal C240H90O4NS for high-grade Anthracite Coal is divided into 4 ranks: (1) Anthracite (2) Bituminous (3) Sub-bituminous (4) Lignite Source: http://cc.msnscache.com/cache.aspx?q=4929705428518&lang=en-US&mkt=en-US&FORM=CVRE8 3 Indiana Center for Coal Technology Research CCTR BITUMINOUS COAL Bituminous Coal: Great pressure results in the creation of bituminous, or “soft” coal. This is the type most commonly used for electric power generation in the U.S. It has a higher heating value than either lignite or sub-bituminous, but less than that of anthracite. Bituminous coal -

Carbon Nanotubes: Synthesis, Integration, and Properties
Acc. Chem. Res. 2002, 35, 1035-1044 Carbon Nanotubes: Synthesis, Integration, and Properties HONGJIE DAI* Department of Chemistry, Stanford University, Stanford, California 94305 Received January 23, 2002 ABSTRACT Synthesis of carbon nanotubes by chemical vapor deposition over patterned catalyst arrays leads to nanotubes grown from specific sites on surfaces. The growth directions of the nanotubes can be controlled by van der Waals self-assembly forces and applied electric fields. The patterned growth approach is feasible with discrete catalytic nanoparticles and scalable on large wafers for massive arrays of novel nanowires. Controlled synthesis of nano- tubes opens up exciting opportunities in nanoscience and nano- technology, including electrical, mechanical, and electromechanical properties and devices, chemical functionalization, surface chem- istry and photochemistry, molecular sensors, and interfacing with soft biological systems. Introduction Carbon nanotubes represent one of the best examples of novel nanostructures derived by bottom-up chemical synthesis approaches. Nanotubes have the simplest chemi- cal composition and atomic bonding configuration but exhibit perhaps the most extreme diversity and richness among nanomaterials in structures and structure-prop- erty relations.1 A single-walled nanotube (SWNT) is formed by rolling a sheet of graphene into a cylinder along an (m,n) lattice vector in the graphene plane (Figure 1). The (m,n) indices determine the diameter and chirality, which FIGURE 1. (a) Schematic honeycomb structure of a graphene sheet. Single-walled carbon nanotubes can be formed by folding the sheet are key parameters of a nanotube. Depending on the along lattice vectors. The two basis vectors a1 and a2 are shown. chirality (the chiral angle between hexagons and the tube Folding of the (8,8), (8,0), and (10,-2) vectors lead to armchair (b), axis), SWNTs can be either metals or semiconductors, with zigzag (c), and chiral (d) tubes, respectively. -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1. -

Carbon Nanostructures – from Molecules to Functionalised Materials
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1537 Carbon Nanostructures – from Molecules to Functionalised Materials Fullerene-Ferrocene Oligomers, Graphene Modification and Deposition MICHAEL NORDLUND ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6214 ISBN 978-91-513-0019-1 UPPSALA urn:nbn:se:uu:diva-327189 2017 Dissertation presented at Uppsala University to be publicly examined in A1:107a, BMC, Husargatan 3, Uppsala, Friday, 22 September 2017 at 09:15 for the degree of Doctor of Philosophy. The examination will be conducted in English. Faculty examiner: Professor Mogens Brøndsted Nielsen (Copenhagen University, Department of chemistry). Abstract Nordlund, M. 2017. Carbon Nanostructures – from Molecules to Functionalised Materials. Fullerene-Ferrocene Oligomers, Graphene Modification and Deposition. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 1537. 64 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0019-1. The work described in this thesis concerns development, synthesis and characterisation of new molecular compounds and materials based on the carbon allotropes fullerene (C60) and graphene. A stepwise strategy to a symmetric ferrocene-linked dumbbell of fulleropyrrolidines was developed. The versatility of this approach was demonstrated in the synthesis of a non- symmetric fulleropyrrolidine-ferrocene-tryptophan triad. A new tethered bis-aldehyde, capable of regiospecific bis-pyrrolidination of a C60-fullerene in predominantly trans fashion, was designed, synthesised and reacted with glycine and C60 to yield the desired N-unfunctionalised bis(pyrrolidine)fullerene. A catenane dimer composed of two bis(pyrrolidine)fullerenes was obtained as a minor co-product. From the synthesis of the N-methyl analogue, the catenane dimer could be separated from the monomeric main product and fully characterised by NMR spectroscopy. -

Introduction to Phase Diagrams*
ASM Handbook, Volume 3, Alloy Phase Diagrams Copyright # 2016 ASM InternationalW H. Okamoto, M.E. Schlesinger and E.M. Mueller, editors All rights reserved asminternational.org Introduction to Phase Diagrams* IN MATERIALS SCIENCE, a phase is a a system with varying composition of two com- Nevertheless, phase diagrams are instrumental physically homogeneous state of matter with a ponents. While other extensive and intensive in predicting phase transformations and their given chemical composition and arrangement properties influence the phase structure, materi- resulting microstructures. True equilibrium is, of atoms. The simplest examples are the three als scientists typically hold these properties con- of course, rarely attained by metals and alloys states of matter (solid, liquid, or gas) of a pure stant for practical ease of use and interpretation. in the course of ordinary manufacture and appli- element. The solid, liquid, and gas states of a Phase diagrams are usually constructed with a cation. Rates of heating and cooling are usually pure element obviously have the same chemical constant pressure of one atmosphere. too fast, times of heat treatment too short, and composition, but each phase is obviously distinct Phase diagrams are useful graphical representa- phase changes too sluggish for the ultimate equi- physically due to differences in the bonding and tions that show the phases in equilibrium present librium state to be reached. However, any change arrangement of atoms. in the system at various specified compositions, that does occur must constitute an adjustment Some pure elements (such as iron and tita- temperatures, and pressures. It should be recog- toward equilibrium. Hence, the direction of nium) are also allotropic, which means that the nized that phase diagrams represent equilibrium change can be ascertained from the phase dia- crystal structure of the solid phase changes with conditions for an alloy, which means that very gram, and a wealth of experience is available to temperature and pressure.