Investigation of Biological Activity of Soil Fungal Extracts and LC/MS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development and Evaluation of Rrna Targeted in Situ Probes and Phylogenetic Relationships of Freshwater Fungi
Development and evaluation of rRNA targeted in situ probes and phylogenetic relationships of freshwater fungi vorgelegt von Diplom-Biologin Christiane Baschien aus Berlin Von der Fakultät III - Prozesswissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktorin der Naturwissenschaften - Dr. rer. nat. - genehmigte Dissertation Promotionsausschuss: Vorsitzender: Prof. Dr. sc. techn. Lutz-Günter Fleischer Berichter: Prof. Dr. rer. nat. Ulrich Szewzyk Berichter: Prof. Dr. rer. nat. Felix Bärlocher Berichter: Dr. habil. Werner Manz Tag der wissenschaftlichen Aussprache: 19.05.2003 Berlin 2003 D83 Table of contents INTRODUCTION ..................................................................................................................................... 1 MATERIAL AND METHODS .................................................................................................................. 8 1. Used organisms ............................................................................................................................. 8 2. Media, culture conditions, maintenance of cultures and harvest procedure.................................. 9 2.1. Culture media........................................................................................................................... 9 2.2. Culture conditions .................................................................................................................. 10 2.3. Maintenance of cultures.........................................................................................................10 -

Fungal Communities in Archives: Assessment Strategies and Impact on Paper Con- Servation and Human Health Fungal
Ana Catarina Martiniano da Silva Pinheiro Licenciada em Conservação e Restauro pela Universidade Nova de Lisboa Licenciada em Ciências Farmacêuticas pela Universidade de Lisboa [Nome completo do autor] [Nome completo do autor] [Habilitações Académicas] Fungal Communities in Archives: Assessment Strategies and Impact on Paper [Nome completo do autor] [Habilitações Académicas] Conservation and Human Health Dissertação para obtenção do Grau de Doutor em [Habilitações Académicas] Ciências[Nome da completoConservação do autor] pela Universidade Nova de Lisboa, Faculdade de Ciências e Tecnologia [NomeDissertação complet parao obtençãodo autor] do[Habilitações Grau de Mestre Académicas] em [Engenharia Informática] Orient ador: Doutora Filomena Macedo Dinis, Professor Auxiliar com Nomeação De- finitiva, DCR, FCT-UNL Co-orientadores:[Nome completo Doutora doLaura autor] Rosado, [Habilitações Instituto Nacional Académicas] de Saúde Doutor Ricardo Jorge I.P. Doutora Valme Jurado, Instituto de Recursos Naturales y Agrobiología, CSIC [Nome completo do autor] [Habilitações Académicas] Júri Presidente Prof. Doutor Fernando Pina Arguentes Doutor António Manuel Santos Carriço Portugal, Professor Auxiliar Doutor Alan Phillips, Investigador Vogais Doutora Maria Inês Durão de Carvalho Cordeiro, Directora da Biblioteca Nacional Doutora Susana Marta Lopes Almeida, Investigadora Auxiliar iii October, 2014 Fungal Communities in Archives: Assessment Strategies and Impact on Paper Con- servation and Human Health Fungal Copyright © Ana Catarina Martiniano da Silva -
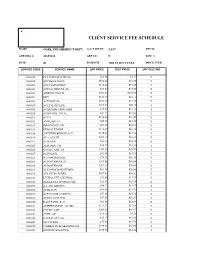
Oakland Sheriff's Dept Fee Schedule 02.09
CLIENT SERVICE FEE SCHEDULE NAME: OAKLAND SHERIFF'S DEPT ACCT SETUP: 2/1/87 REP ID: GRP BILL #: 22857610 GRP CD: N DISC %: FS ID: 01 FS DESCR: 2003 CLIENT FEES PRICE TYPE: SERVICE CODE SERVICE NAME UPF PRICE TEST PRICE UPF DISC IND 0000018 PLT SODIUM CITRATE $12.86 $4.37 Y 0000022 APC RESISTANCE $101.62 $34.55 Y 0000201 ACETAMINOPHEN $110.24 $37.48 Y 0000202 ACETALDEHYDE (B) $73.00 $73.00 N 0000203 ARSENIC,TISSUE $302.00 $302.00 N 0000204 DHT $182.70 $62.12 Y 0000205 ACETONE (B) $102.20 $34.75 Y 0000206 ACETYLCHOLINE $165.00 $56.10 Y 0000208 ACID PHOS, PROS, IMM $35.60 $12.10 Y 0000210 ACID PHOS, TOTAL $37.22 $12.65 Y 0000211 ACTH $110.00 $37.40 Y 0000212 AMYLASE (U) $45.45 $15.45 Y 0000213 IMMUNOFIX, UR $67.39 $22.91 Y 0000214 ETHOSUXIMIDE $112.07 $38.10 Y 0000216 ANTITHROMBIN III ACT $170.46 $57.96 Y 0000219 ALA, QUANT $107.10 $36.41 Y 0000223 ALBUMIN $22.88 $22.88 N 0000224 ALBUMIN, CSF $36.25 $12.33 Y 0000225 CYCLIC AMP, UR $205.80 $69.97 Y 0000227 ALDOLASE $32.08 $10.91 Y 0000228 A-2-MACROGLOB. $78.75 $26.78 Y 0000229 ALDOSTERONE (U) $251.58 $85.54 Y 0000230 ALDOSTERONE $207.10 $70.41 Y 0000231 ALK PHOS ISOENZYMES $61.34 $20.86 Y 0000232 AFP, FLUID W/RFX $107.40 $36.52 Y 0000233 LEUKOCYTE ALK PHOS $34.60 $11.76 Y 0000234 ALKALINE PHOSPHATASE $22.88 $22.88 N 0000235 A-1-ANTITRYPSN $40.22 $13.67 Y 0000236 AMIKACIN $99.91 $33.97 Y 0000237 AFP,TUMOR (CHIRON) $53.86 $18.31 Y 0000238 AMINO ACID SCR $87.15 $29.63 Y 0000240 RAST PANEL #103 $62.00 $62.00 N 0000241 AMPHETAMINE GC/MS $111.70 $37.98 Y 0000242 CYCLIC AMP $205.80 $69.97 Y 0000243 AMYLASE $16.42 $5.58 Y 0000248 HACKBERRY IGE $35.40 $35.40 N 0000249 ANA W/RFX $55.00 $18.70 Y 0000250 *AMIKACIN,PEAK&TROUGH $199.83 $67.94 Y 0000251 ANDROSTENEDIONE $180.65 $61.42 Y 0000252 ANTI-DIUR. -

Endophytic Fungi: Treasure for Anti-Cancerous Compounds
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 8, Issue 8, 2016 Review Article ENDOPHYTIC FUNGI: TREASURE FOR ANTI-CANCEROUS COMPOUNDS ANAND DILIP FIRODIYAa*, RAJESH KUMAR TENGURIAb aCSRD, Peoples University, Bhopal 462037, Madhya Pradesh, India, bDepartment of Botany, Govt. PG College, Rajgarh 496551, Madhya Pradesh, India Email: [email protected] Received: 22 Apr 2016 Revised and Accepted: 20 June 2016 ABSTRACT Endophytic fungi that live asymptomatically inside the plant tissues have novel bioactive metabolites exhibiting a variety of biological activities, especially against cancer. This review highlights the research progress on the production of anticancer compounds by endophytic fungi from 1990- 2015. Anticancer activity is generally associated with the cytotoxicity of the compounds present in the endophytic fungi. The ubiquitous nature of endophytic fungi synthesise diverse chemicals with promising anticancer activity from either their original host or related species. Modification in fermentation parameters and genetic insight of endophytes may produce novel anti-cancerous compounds. Keywords: Cancer, Medicinal plants, Secondary metabolites © 2016 The Authors. Published by Innovare Academic Sciences Pvt Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/) INTRODUCTION endophytic fungi detectable by high-performance liquid chromate- graphy, nuclear magnetic resonance, mass spectrophotometer and The interest in the biogenic medicines has revived throughout the X-ray crystallography and its cytotoxicity of the bioactive world, as the increase in awareness of the health hazards and compounds against cancer cell lines. The compounds with potential toxicity associated with the random use of synthetic drugs and application were also considered in the selection of antitumor antibiotics [1]. -

And Mushroom-Associated Alkaloids from Two Behavior Modifying
bioRxiv preprint doi: https://doi.org/10.1101/375105; this version posted December 18, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Psychoactive plant- and mushroom-associated alkaloids from two behavior modifying 2 cicada pathogens 3 4 Greg R. Boyce1, Emile Gluck-Thaler2, Jason C. Slot2, Jason E. Stajich3, William J. Davis4, Tim 5 Y. James4, John R. Cooley5, Daniel G. Panaccione1, Jørgen Eilenberg6, Henrik H. De Fine Licht6, 6 Angie M. Macias1, Matthew C. Berger1, Kristen L. Wickert1, Cameron M. Stauder1, Ellie J. 7 Spahr1, Matthew D. Maust1, Amy M. Metheny1, Chris Simon5, Gene Kritsky7, Kathie T. Hodge8, 8 Richard A. Humber8,9, Terry Gullion10, Dylan P. G. Short11, Teiya Kijimoto1, Dan Mozgai12, 9 Nidia Arguedas13, Matt T. Kasson1,* 10 11 1Division of Plant and Soil Sciences, West Virginia University, Morgantown, West Virginia, 12 26506, USA. 13 2Department of Plant Pathology, The Ohio State University, Columbus, Ohio 43210, USA. 14 3Department of Microbiology and Plant Pathology and Institute for Integrative Genome Biology, 15 University of California, Riverside, California 92521, USA. 16 4Department of Ecology and Evolution, University of Michigan, Ann Arbor, Michigan 48109, 17 USA. 18 5Department of Ecology and Evolutionary Biology, University of Connecticut, Storrs, 19 Connecticut 06269, USA. 20 6Department of Plant and Environmental Science, University of Copenhagen, Denmark. 21 7Department of Biology, Mount St. Joseph University, Cincinnati, Ohio 45233, USA. 22 8Plant Pathology & Plant-Microbe Biology, School of Integrative Plant Science, Cornell 23 University, Ithaca, New York 14853, USA. -

The Phylogeny of Plant and Animal Pathogens in the Ascomycota
Physiological and Molecular Plant Pathology (2001) 59, 165±187 doi:10.1006/pmpp.2001.0355, available online at http://www.idealibrary.com on MINI-REVIEW The phylogeny of plant and animal pathogens in the Ascomycota MARY L. BERBEE* Department of Botany, University of British Columbia, 6270 University Blvd, Vancouver, BC V6T 1Z4, Canada (Accepted for publication August 2001) What makes a fungus pathogenic? In this review, phylogenetic inference is used to speculate on the evolution of plant and animal pathogens in the fungal Phylum Ascomycota. A phylogeny is presented using 297 18S ribosomal DNA sequences from GenBank and it is shown that most known plant pathogens are concentrated in four classes in the Ascomycota. Animal pathogens are also concentrated, but in two ascomycete classes that contain few, if any, plant pathogens. Rather than appearing as a constant character of a class, the ability to cause disease in plants and animals was gained and lost repeatedly. The genes that code for some traits involved in pathogenicity or virulence have been cloned and characterized, and so the evolutionary relationships of a few of the genes for enzymes and toxins known to play roles in diseases were explored. In general, these genes are too narrowly distributed and too recent in origin to explain the broad patterns of origin of pathogens. Co-evolution could potentially be part of an explanation for phylogenetic patterns of pathogenesis. Robust phylogenies not only of the fungi, but also of host plants and animals are becoming available, allowing for critical analysis of the nature of co-evolutionary warfare. Host animals, particularly human hosts have had little obvious eect on fungal evolution and most cases of fungal disease in humans appear to represent an evolutionary dead end for the fungus. -

Coprophilous Fungal Community of Wild Rabbit in a Park of a Hospital (Chile): a Taxonomic Approach
Boletín Micológico Vol. 21 : 1 - 17 2006 COPROPHILOUS FUNGAL COMMUNITY OF WILD RABBIT IN A PARK OF A HOSPITAL (CHILE): A TAXONOMIC APPROACH (Comunidades fúngicas coprófilas de conejos silvestres en un parque de un Hospital (Chile): un enfoque taxonómico) Eduardo Piontelli, L, Rodrigo Cruz, C & M. Alicia Toro .S.M. Universidad de Valparaíso, Escuela de Medicina Cátedra de micología, Casilla 92 V Valparaíso, Chile. e-mail <eduardo.piontelli@ uv.cl > Key words: Coprophilous microfungi,wild rabbit, hospital zone, Chile. Palabras clave: Microhongos coprófilos, conejos silvestres, zona de hospital, Chile ABSTRACT RESUMEN During year 2005-through 2006 a study on copro- Durante los años 2005-2006 se efectuó un estudio philous fungal communities present in wild rabbit dung de las comunidades fúngicas coprófilos en excementos de was carried out in the park of a regional hospital (V conejos silvestres en un parque de un hospital regional Region, Chile), 21 samples in seven months under two (V Región, Chile), colectándose 21 muestras en 7 meses seasonable periods (cold and warm) being collected. en 2 períodos estacionales (fríos y cálidos). Un total de Sixty species and 44 genera as a total were recorded in 60 especies y 44 géneros fueron detectados en el período the sampling period, 46 species in warm periods and 39 de muestreo, 46 especies en los períodos cálidos y 39 en in the cold ones. Major groups were arranged as follows: los fríos. La distribución de los grandes grupos fue: Zygomycota (11,6 %), Ascomycota (50 %), associated Zygomycota(11,6 %), Ascomycota (50 %), géneros mitos- mitosporic genera (36,8 %) and Basidiomycota (1,6 %). -
Fungal Endophytes As Efficient Sources of Plant-Derived Bioactive
microorganisms Review Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and Their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges Archana Singh 1,2, Dheeraj K. Singh 3,* , Ravindra N. Kharwar 2,* , James F. White 4,* and Surendra K. Gond 1,* 1 Department of Botany, MMV, Banaras Hindu University, Varanasi 221005, India; [email protected] 2 Department of Botany, Institute of Science, Banaras Hindu University, Varanasi 221005, India 3 Department of Botany, Harish Chandra Post Graduate College, Varanasi 221001, India 4 Department of Plant Biology, Rutgers University, New Brunswick, NJ 08901, USA * Correspondence: [email protected] (D.K.S.); [email protected] (R.N.K.); [email protected] (J.F.W.); [email protected] (S.K.G.) Abstract: Fungal endophytes are well-established sources of biologically active natural compounds with many producing pharmacologically valuable specific plant-derived products. This review details typical plant-derived medicinal compounds of several classes, including alkaloids, coumarins, flavonoids, glycosides, lignans, phenylpropanoids, quinones, saponins, terpenoids, and xanthones that are produced by endophytic fungi. This review covers the studies carried out since the first report of taxol biosynthesis by endophytic Taxomyces andreanae in 1993 up to mid-2020. The article also highlights the prospects of endophyte-dependent biosynthesis of such plant-derived pharma- cologically active compounds and the bottlenecks in the commercialization of this novel approach Citation: Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. in the area of drug discovery. After recent updates in the field of ‘omics’ and ‘one strain many Fungal Endophytes as Efficient compounds’ (OSMAC) approach, fungal endophytes have emerged as strong unconventional source Sources of Plant-Derived Bioactive of such prized products. -

Fungal Endophytes: Promising Tools for Pharmaceutical Science
Int. J. Pharm. Sci. Rev. Res., 25(2), Mar – Apr 2014; Article No. 25, Pages: 128-138 ISSN 0976 – 044X Research Article Fungal Endophytes: Promising Tools for Pharmaceutical Science Pramod Kumar Pandey1,2, Siddhartha Singh1, Raj Naraian Singh Yadav2, Amit Kumar Singh1*, M. Chandra Kumar Singh1 1Department of Basic Science and Humanities, College of Horticulture and Forestry, Central Agricultural University, Pasighat, Arunachal Pradesh, India. 2Centre for Studies in Biotechnology, Dibrugarh University, Dibrugarh, Assam, India. *Corresponding author’s E-mail: [email protected] Accepted on: 27-01-2014; Finalized on: 31-03-2014. ABSTRACT Fungal endophytes are microorganisms that internally infect living plant tissues without causing any visible symptom of infection, and live in mutualistic relationship with plants for at least a part of their life cycle. Every plant in the world is reservoir of one or more number of endophytes. In recent year’s special attention have been made towards endophytic fungi because of their ability to synthesize several novel bioactive compounds not previously known to biological system which are important for pharmaceutical, agricultural and industrial sector. This review describes information on endophyte diversity, as well as production of secondary metabolites with special emphasis on anti cancerous, antimicrobial, antiviral, antibiotics along with huge number of other secondary metabolites for commercial exploitation in pharmaceutical and medical field. Furthermore, the chemical potential of endophytic fungi for drug discovery will be discussed with focus on the detection of pharmaceutically valuable plant constituents as products of fungal biosynthesis in recent years. At present a huge world population is suffering from the problem caused by drug resistant microbes (bacteria, parasitic protozoans and fungus) which decreases the efficiency of synthetic drugs. -

ZANCO Journal of Pure and Applied Sciences Seed-Borne Fungi of Durum Wheat (Triticum Durum Desf) Cultivars Grown in Duhok Provin
ZANCO Journal of Pure and Applied Sciences The official scientific journal of Salahaddin University-Erbil ZJPAS (2017), 29 (s4); 300-309 http://dx.doi.org/10.21271/ZJPAS.29.s4.34 Seed-borne fungi of durum wheat (Triticum durum Desf) cultivars grown in Duhok province, Kurdistan Region, Iraq Samir K. Abdullah1* and Helben I. M. Atroshi2 1 Biology Department, Faculty of Science, University of Zakho 2 Plant Protection Department, College of Agriculture and Forestry, Duhok University, Iraq A R T I C L E I N F O A B S T R A C T Article History: The study was carried out to investigate the seed borne fungi associated with Received: 01/06/2017 different cultivars of durum wheat (Triticum durum Desf.) grown in Duhok Accepted: 05/08/2017 province, Kurdistan region, Iraq. A total of 12 seed samples were surveyed for Published: 20/12/2017 the associated mycoflora using standard agar plate, blotter paper and freezing Keywords: methods. A total of 21 fungal species assigned to 14 genera were identified. Durum wheat, The most common genera in descending order of their isolation frequency were Seed-borne fungi, Cladosporium, Penicillium, Alternaria, Aspergillus, Ulocladium, Arthrinium Iraq and Chaetomium. Alternaria alternata,Cladosporium herbarum,Aspergillus flavus, A.niger, and Arthrinium phaeospermum were the most frequent species. *Corresponding Author: Ascad cultivar showed the highest number of detected species (13 species), Samir K. Abdullah whereas the lowest number (7 species) were isolated from Sham 3 cultivar. [email protected] Ascad cultivar (sample 2). Sham 3 (sample 5) and Simeto (sample 7) showed the highest mean percentage contamination (71.0%, 68.5% and 57.0%) respectively. -

COMPARATIVE BIOLOGICAL EVALUATION of SIX ENDOPHYTIC FUNGI ISOLATED from VINCA ROSEA LEAVES Ahmed M
Az. J. Pharm Sci. Vol. 59, March, 2019. 137 COMPARATIVE BIOLOGICAL EVALUATION OF SIX ENDOPHYTIC FUNGI ISOLATED FROM VINCA ROSEA LEAVES Ahmed M. Metwaly*1, Mohamed A. Ashour1, Shabana Khan2, Guoyi Ma2, Hazem A. Kadry1, Atef A. El-Hela1, Abd-Elsalam I. Mohammad1, Stephen J. Cutler3 and Samir A. Ross2 1Department of Pharmacognosy, Faculty of Pharmacy, Al-Azhar University, Cairo, Egypt 2 Department of Biomolecular Science, National Center for Natural Products Research, University of Mississippi, Oxford, MS 38677, USA 3Department of Medicinal Chemistry, School of Pharmacy, the University of Mississippi, Oxford, MS 38677, USA *Corresponding authors: [email protected] Abstract In this study, a total of six endophytic fungi have been isolated from Vinca rosea (Apocynaceae) leaves growing in Egypt. The isolated fungi were identified morphologically and microscopically up to species to be; Alternaria phragmospora, Aspergillus awamori, Penicillium duclauxii, Penicillium melinii, Nigrospora sphaerica and Mucor ramosissimus. The extracts of the all identified fungi were screened biologically for antileukemic, cytotoxic, antimalarial, antileishmanial, antimicrobial, antioxidant and anti-inflammatory activities as well as for cannabinoid and opioid receptor binding affinities. Out of the six examined fungal extracts four exhibited promising antimalarial activities, four showed modeate antileukemic activities and two of them exhibited cytotoxic activities, three showed antioxidant activities, three exhibited anti-inflammatory activities, and the only one showed an antifungal activity. Alternaria phragmospora was the most active antimalarial agent inhibiting Plasmodium falciparum D6 and W2 clones with IC50 values of 1.9 and 2.1 μg/mL, respectively. Alternaria phragmospora extract was subjected to liquid-liquid partition using 90% MeOH, hexane then BuOH and H2O. -

Fungal Biodiversity in Extreme Environments and Wood Degradation Potential
http://waikato.researchgateway.ac.nz/ Research Commons at the University of Waikato Copyright Statement: The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). The thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: Any use you make of these documents or images must be for research or private study purposes only, and you may not make them available to any other person. Authors control the copyright of their thesis. You will recognise the author’s right to be identified as the author of the thesis, and due acknowledgement will be made to the author where appropriate. You will obtain the author’s permission before publishing any material from the thesis. Fungal biodiversity in extreme environments and wood degradation potential A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biological Sciences at The University of Waikato by Joel Allan Jurgens 2010 Abstract This doctoral thesis reports results from a multidisciplinary investigation of fungi from extreme locations, focusing on one of the driest and thermally broad regions of the world, the Taklimakan Desert, with comparisons to polar region deserts. Additionally, the capability of select fungal isolates to decay lignocellulosic substrates and produce degradative related enzymes at various temperatures was demonstrated. The Taklimakan Desert is located in the western portion of the People’s Republic of China, a region of extremes dominated by both limited precipitation, less than 25 mm of rain annually and tremendous temperature variation.