Building a Stem Cell-Based Primate Uterus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter V FOLLICULAR DYNAMICS and REPRODUCTIVE
Chapter V FOLLICULAR DYNAMICS AND REPRODUCTIVE TECHNOLOGIES IN BUFFALO Giuseppina Maria Terzano Istituto Sperimentale per la Zootecnia (Animal Production Research Institute) Via Salaria 31, 00016 Monterotondo (Rome), Italy The general characteristics of reproduction like seasonality, cyclicity and ovulation differ widely in mammals for the following reasons: a) reproductive activity may take place during the whole year or at defined seasons, according to the species and their adaptation to environmental conditions; thus, photoperiod plays a determinant role in seasonal breeders such as rodents, carnivores and ruminants (sheep, goats, buffaloes, deer, etc.,). An extreme situation is observed in foxes with only one ovulation per year, occurring in January or February; b) mammals may be distinguished according to the absence or presence of spontaneous ovulations: in the first group of mammals ( rabbits, hares, cats, mink, camels, Llama), the ovulation is induced by mating and cyclicity is not obvious; in the second group, ovulation occurs spontaneously in each cycle, separating the follicular phase from the luteal phase; c) the length of cycles is quite different among species: small rodents have short cycles of four or five days, farm animals and primates have longer cycles (sheep: 17 days; cow, goat, buffalo, horse and pig: 21 days; primates: 28 days), and dogs are characterized by long cycles of six to seven months, including a two month luteal phase (Concannon, 1993); d) ovulation rates differ widely among species and breeds within a given species: in sheep for example, Merinos d'Arles or Ile-de-France breeds have only one ovulation per cycle, whereas average rates of two to four ovulations per cycle are observed in prolific breeds like Romanov or Finn (Land et al., 1973). -

Oogenesis in Mammals
OOGENESIS IN MAMMALS In contrast to most other vertebrates , mammals do not replenish the stores of oocytes present in the ovary at birth. At birth the human ovaries contain about 1 million oocytes ( many of which are already degenerating) that have been arrested in the diplotene stage of the first meiotic division . These oocytes are already surrounded by a layer of follicular cells or granulosa cells , and the complex of ovum and its surrounding cellular investments is known as a follicle . Of all the germ cells present in the ovary ,only about 400 (one per menstrual cycle) will reach maturity and become ovulated. The remainder develop to varying degrees and then undergo atresia (degeneration). Oocytes first become associated with follicular cells in the late fetal period , when they are going through early prophase of the first meiotic division . The primary oocyte (so called because it is undergoing the first meiotic division ) plus its incomplete covering of flattened follicular cells is called a primordial follicle . According to Gougeon (1993) a follicle passes through three major phases on its way to ovulation. The first phase is characterized by a large pool of nongrowing follicles , approximately 500,000 per ovary at birth. In this pool are primordial follicles, which develop into primary follicles by surrounding themselves with a complete single layer of cuboidal follicular cells . By this time , the oocytes have entered the first period of meiotic arrest , the diplotene stage . In human , essentially all oocytes , unless Page 1 of 5 : SEM-2 (GEN ) , Unit#6 : OOGENESIS : Pritha Mondal they degenerate ,remain arrested in the diplotene stage until puberty ; some will not progress past the diplotene stage until the woman’s last reproductive cycle (age 45 to 55 years). -

The Effectiveness and Safety of the Early Follicular Phase Full-Dose Down
The effectiveness and safety of the early follicular phase full-dose down- regulation protocol for controlled ovarian hyperstimulation: a randomized, paralleled controlled, multicenter trial 2018-12-29 Background Since the first “tube baby”, Louise Brown, was born in the United Kingdom in 1978, many infertile couples have been benefitted from in vitro fertilization and embryo transfer (IVF-ET) and intracytoplasmic sperm injection (ICSI). It is reported that there are over 5 million babies born with the help of assisted reproductive technology (ART). According to the 2015 national data published by Human Fertility and Embryology Authority (HFEA, 48,147 women received 61,726 IVF/ICSI cycles and gave birth to 17,041 newborns [1]. In the United States, 169,602 IVF/ICSI cycles were performed in 2014 and 68,791 tubal babies were born [2]. China has a huge population base, and therefore has a substantial number of infertile couples. Although a late starter, China is developing rapidly in ART and playing a more and more important role in the area of reproductive medicine. In spite of the continuous development in ART, so far, the overall success rate of IVF/ICSI is still hovering around 25-40%. The live birth rate per stimulated cycle is 25.6% in the UK in 2015, fluctuating from 1.9% in women aged 45 and elder to 32.2% in women younger than 35 years old [1]. The IVF/ICSI success rate in 2014 in the US is similar [2]. In China, according to the data submitted by 115 reproductive medicine centers on the ART data reporting system developed by Chinese Society of Reproductive Medicine, the delivery rate is about 40% [3]. -

Variability in the Length of Menstrual Cycles Within and Between Women - a Review of the Evidence Key Points
Variability in the Length of Menstrual Cycles Within and Between Women - A Review of the Evidence Key Points • Mean cycle length ranges from 27.3 to 30.1 days between ages 20 and 40 years, follicular phase length is 13-15 days, and luteal phase length is less variable and averages 13-14 days1-3 • Menstrual cycle lengths vary most widely just after menarche and just before menopause primarily as cycles are anovulatory 1 • Mean length of follicular phase declines with age3,11 while luteal phase remains constant to menopause8 • The variability in menstrual cycle length is attributable to follicular phase length1,11 Introduction Follicular and luteal phase lengths Menstrual cycles are the re-occurring physiological – variability of menstrual cycle changes that happen in women of reproductive age. Menstrual cycles are counted from the first day of attributable to follicular phase menstrual flow and last until the day before the next onset of menses. It is generally assumed that the menstrual cycle lasts for 28 days, and this assumption Key Points is typically applied when dating pregnancy. However, there is variability between and within women with regard to the length of the menstrual cycle throughout • Follicular phase length averages 1,11,12 life. A woman who experiences variations of less than 8 13-15 days days between her longest and shortest cycle is considered normal. Irregular cycles are generally • Luteal phase length averages defined as having 8 to 20 days variation in length of 13-14 days1-3 cycle, whereas over 21 days variation in total cycle length is considered very irregular. -

Effects of Caffeine, Alcohol and Smoking on Fertility
Pre-Conception Health Special Interest Group Effects of caffeine, alcohol and smoking on fertility There is an increasing body of evidence that health behaviours affect fertility. As most health behaviours can be modified, providing advice and support in making healthy changes can promote fertility. The evidence relating to the effects on fertility of caffeine, alcohol consumption and smoking is reviewed here. Your Fertility is a national public education campaign funded by the Australian Government Department of Health and Ageing under the Family Planning Grants Program. 1 Updated October 2015 Pre-Conception Health Special Interest Group Effects of caffeine, alcohol and smoking on fertility Evidence review Caffeine Smoking Caffeine is widely consumed as it is present in coffee, tea, some soft drinks There is strong evidence that smoking adversely affects male and female and chocolate. Some evidence suggests that the consumption of caffeine, fertility. Smokers are more likely to be infertile [7, 20-21] and women with a possible dose-response effect, may prolong the time to pregnancy who are exposed to smoking take longer to conceive [22]. Furthermore, and affect the health of a developing foetus, although the mechanism for maternal smoking increases the risk of low birth weight and birth defects this is unclear. Caffeine may affect ovulation and corpus luteum functioning [23] and women who smoke reach menopause earlier than non-smokers through alterations to hormone levels [1] and has been shown to be associated [24]. Smoking can also damage sperm DNA. Heavy smoking (≥20 with elevated early follicular E2 levels in females [2]. Although some studies cigarettes per day) by fathers at the time of conception increases the have found a positive relationship between caffeine consumption and time child’s risk of childhood leukaemia and shortens reproductive lifespan to conception [3-6], study results are inconsistent and should be interpreted of daughters [25-26]. -

Research Journal of Pharmaceutical, Biological and Chemical Sciences
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Alcohol Affects Pregnancy by Hypothalamic- Pituitary-Gonadal Axis Derangement. Nwangwa EK1*, Ekhoye EI1, Naiho AO1, and Ekene N2. 1Department of Physiology, 2Department of Pharmacology and Therapeutics Faculty of Basic Medical Sciences, College of Health Sciences, Delta State University, P.M.B. 001 Abraka. Delta State. Nigeria. ABSTRACT The present study investigated the deleterious effects of alcohol exposure on reproductive hormones during pregnancy. Forty Wistar albino rats (30 female & 10 male) were mated and randomized into 6 groups (n=5) and then administered with 25% alcohol daily through orogastric cannula from gestation day 0 to day 7 (Group 1), day 8 to day 14 (group 2) and day 15 to 21 (group 3). Group 4, 5 and 6 were control for 1, 2 and 3 respectively. At the end of each trimester, five rats were sacrificed from each experimental and corresponding control group and blood samples collected for FSH, LH, oestrogen and progesterone assay. The male rats were used for mating purposes only. One way analysis of Variance was used to compare means, p-value < 0.05 was considered significant. The result showed that alcohol decreased the FSH, LH, estrogen and progesterone levels during the course of the pregnancy with significance (p<0.05) especially in the third trimester. It was expected that there will be a rise in level of FSH and LH in response to decreasing oestrogen and progesterone level but that was not the case here This study therefore demonstrates that alcohol exposure adversely affects pregnancy through decrease in hormones that help in maintaining pregnancy especially in the third trimester possibly due to a derangement in the Hypothalamic- Pituitary-Gonadal (HPG) axis. -
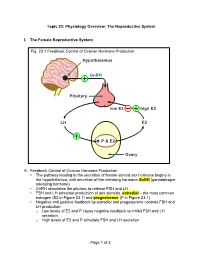
Of 3 Topic 23: Physiology Overview: the Reproductive System I. The
Topic 23: Physiology Overview: The Reproductive System I. The Female Reproductive System Fig. 23.1 Feedback Control of Ovarian Hormone Production Hypothalamus GnRH Pituitary low E2 high E2 LH E2 é P & E2 Ovary A. Feedback Control of Ovarian Hormone Production • The pathway leading to the secretion of female steroid sex hormone begins in the hypothalamus, with secretion of the releasing hormone GnRH (gonadotropin- releasing hormone) • GnRH stimulates the pituitary to release FSH and LH • FSH and LH stimulate production of sex steroids, estradiol – the most common estrogen (E2 in Figure 23.1) and progesterone (P in Figure 23.1) • Negative and positive feedback by estradiol and progesterone controls FSH and LH production: o Low levels of E2 and P cause negative feedback to inhibit FSH and LH secretion o High levels of E2 and P stimulate FSH and LH secretion Page 1 of 3 PCOL260 23. Physiology Overview: The Reproductive System B. The Menstrual Cycle Figure 23.2 The Menstrual Cycle Ovulation LH FSH Estradiol Relative Blood Level Progesterone 1287 14 21 Days of Menstrual Cycle 1. Follicular phase • The menstrual cycle begins as FSH stimulates development of an ovarian follicle (an egg surrounded by a covering of cells). As this follicle matures, it begins producing a small but steadily rising amount of estradiol. This low level of estradiol causes: o Negative feedback: inhibition of FSH and LH secretion o Growth of the lining of the uterus 2. Ovulation • The amount of estradiol secreted at mid-cycle has increased to the high level that stimulates a surge of FSH and LH release by the pituitary • The LH surge causes ovulation: the follicle ruptures and releases its egg near the opening of its fallopian tube 3. -

Reproductive Cycles in Females
MOJ Women’s Health Review Article Open Access Reproductive cycles in females Abstract Volume 2 Issue 2 - 2016 The reproductive system in females consists of the ovaries, uterine tubes, uterus, Heshmat SW Haroun vagina and external genitalia. Periodic changes occur, nearly every one month, in Faculty of Medicine, Cairo University, Egypt the ovary and uterus of a fertile female. The ovarian cycle consists of three phases: follicular (preovulatory) phase, ovulation, and luteal (postovulatory) phase, whereas Correspondence: Heshmat SW Haroun, Professor of the uterine cycle is divided into menstruation, proliferative (postmenstrual) phase Anatomy and Embryology, Faculty of Medicine, Cairo University, and secretory (premenstrual) phase. The secretory phase of the endometrium shows Egypt, Email [email protected] thick columnar epithelium, corkscrew endometrial glands and long spiral arteries; it is under the influence of progesterone secreted by the corpus luteum in the ovary, and is Received: June 30, 2016 | Published: July 21, 2016 an indicator that ovulation has occurred. Keywords: ovarian cycle, ovulation, menstrual cycle, menstruation, endometrial secretory phase Introduction lining and it contains the uterine glands. The myometrium is formed of many smooth muscle fibres arranged in different directions. The The fertile period of a female extends from the age of puberty perimetrium is the peritoneal covering of the uterus. (11-14years) to the age of menopause (40-45years). A fertile female exhibits two periodic cycles: the ovarian cycle, which occurs in The vagina the cortex of the ovary and the menstrual cycle that happens in the It is the birth and copulatory canal. Its anterior wall measures endometrium of the uterus. -

Endometrium and Embryo Implantation: the Hidden Frontier
FINAL PROGRAM AND ABSTRACT 0 1 0 2 , 5 2 - 4 2 r e b m te ep - S Lyon, France Endometrium and Embryo Implantation: the Hidden Frontier Your Continuing Medical Education Partner www.seronosymposia.org Endometrium and Embryo Implantation: the Hidden Frontier GENERAL INFORMATION VENUE Hilton Lyon 70 Quai Charles De Gaulle 69463 Lyon, France Tel: +33-4-7817-5050 LANGUAGE The official language of this Conference will be English. LOCATION Lyon, city of pairs with its 2 rivers, the Rhône and Saône, and its twin hilly districts, Fourvière “the hill of prayers” and Croix Rousse “the hill of workers”. A city proud of its monuments that cohabit marvelously with very contempor ary buildings. Intimate and open, mysterious and influential, impetuous and nonchalant, the home of the famous bouchon bistros, a capital of gastronomy, surrounded by vineyards known the world over, listed by UNESCO as a World Heritage site, a city famous for the invention of the silk and the birthplace of the inventors of cinema. Serono Symposia International Foundation, Annual Conference on: Endometrium and Embryo Implantation: the Hidden Frontier Lyon, France - September 24-25, 2010 AIM OF THE CONFERENCE Implantation is a complex process which requires the orchestration of a series of events involving both the embryo and the endometrium. Due to the complexity of the mechanisms involved and relative lack of information on some of them, it has been defined “black box” of assisted reproduction. Even with the transfer of high quality embryos, implantation rates still remain relatively low, thus failure of implantation is a major factor which influences negatively ART outcomes. -

REVIEW Impact of the Menstrual Cycle on Determinants of Energy Balance: a Putative Role in Weight Loss Attempts
International Journal of Obesity (2007) 31, 1777–1785 & 2007 Nature Publishing Group All rights reserved 0307-0565/07 $30.00 www.nature.com/ijo REVIEW Impact of the menstrual cycle on determinants of energy balance: a putative role in weight loss attempts L Davidsen, B Vistisen and A Astrup Department of Human Nutrition, Faculty of Life Sciences, University of Copenhagen, Frederiksberg, Denmark Women’s weight and body composition is significantly influenced by the female sex-steroid hormones. Levels of these hormones fluctuate in a defined manner throughout the menstrual cycle and interact to modulate energy homeostasis. This paper reviews the scientific literature on the relationship between hormonal changes across the menstrual cycle and components of energy balance, with the aim of clarifying whether this influences weight loss in women. In the luteal phase of the menstrual cycle it appears that women’s energy intake and energy expenditure are increased and they experience more frequent cravings for foods, particularly those high in carbohydrate and fat, than during the follicular phase. This suggests that the potential of the underlying physiology related to each phase of the menstrual cycle may be worth considering as an element in strategies to optimize weight loss. Studies are needed to assess the weight loss outcome of tailoring dietary recommendations and the degree of energy restriction to each menstrual phase throughout a weight management program, taking these preliminary findings into account. International Journal of Obesity -

Control of Growth and Development of Preantral Follicle: Insights from in Vitro Culture
DOI: 10.21451/1984-3143-AR2018-0019 Proceedings of the 10th International Ruminant Reproduction Symposium (IRRS 2018); Foz do Iguaçu, PR, Brazil, September 16th to 20th, 2018. Control of growth and development of preantral follicle: insights from in vitro culture José Ricardo de Figueiredo1,*, Laritza Ferreira de Lima1, José Roberto Viana Silva2, Regiane Rodrigues Santos3 1Laboratory of Manipulation of Oocytes and Preantral Follicles, Faculty of Veterinary, State University of Ceara, Fortaleza CE, Brazil. 2Biotecnology Nucleus of Sobral (NUBIS), Federal University of Ceara, Sobral, CE, Brazil. 3Schothorst Feed Research, Lelystad, The Netherlands. Abstract interaction among endocrine, paracrine and autocrine factors, which in turn affects the steroidogenesis, The regulation of folliculogenesis involves a angiogenesis, basement membrane turnover, oocyte complex interaction among endocrine, paracrine and growth and maturation as well as follicular atresia autocrine factors. The mechanisms involved in the (reviewed by Atwood and Meethala, 2016). It is well initiation of the growth of the primordial follicle, i.e., known that mammalian ovaries contain from thousands follicular activation and the further growth of primary to millions of follicles, whereby about 90% of them are follicles up to the pre-ovulatory stage, are not well represented by preantral follicles (PFs). The understood at this time. The present review focuses on mechanisms involved in the initiation of growth of the the regulation and development of early stage primordial follicles, i.e., follicular activation and the (primordial, primary, and secondary) folliculogenesis further growth of primary follicles up to the pre- highlighting the mechanisms of primordial follicle ovulatory stage, are not well understood at this time. It activation, growth of primary and secondary follicles is important to emphasize that despite the large number and finally transition from secondary to tertiary of follicles in the ovary, the vast majority follicles. -

Histological Evidence of Alternating Ovulation in Women A
Histological evidence of alternating ovulation in women A. Gougeon and Brigitte Lef\l=e`\vre Physiologie et psychologie de la reproduction humaine, INSERM U-187, 32 rue des Carnets, 92140 Clamart, France Summary. Detailed histological analysis of ovaries obtained from 25 women by ovariectomy for medical reasons demonstrated that the degeneration of the cyclic corpus luteum is a long process, taking several cycles. The ages of the various regressing corpora lutea were identified to permit classification of the side of ovulation during the preceding 4 or 5 cycles. In the 25 women studied, with regular menstrual cycles of 28 \m=+-\3 days (48 cycles), ovulation occurred most often (87\m=.\6%)in an alternating manner. Introduction It is not known whether the single follicle that ovulâtes in each cycle of primates does so by chance in either of the two ovaries or is influenced by the side of ovulation of the preceding cycle. The follicle destined to ovulate and/or the cyclic corpus luteum could influence follicular development in such a way that the selection of the dominant follicle is not by chance. Riihl (1925) suggested that ovulation in women was alternate, but his results, obtained from post-mortem ovaries, were not quantitative or illustrated. The question is also unresolved in non-human primates. Wallach, Virutamasen & Wright (1973) and Clark, Dierschke & Wolf (1978) reported that ovulation occurred by chance in either ovary, uninfluenced by the side of ovulation in the previous cycle in the rhesus monkey. However, Dukelow (1977) and Hodgen (1982) consider that ovulation in the cynomolgus monkey and rhesus monkey, respectively, occurs alternately as long as the cycles are not perturbed.