Endometrium and Embryo Implantation: the Hidden Frontier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter V FOLLICULAR DYNAMICS and REPRODUCTIVE
Chapter V FOLLICULAR DYNAMICS AND REPRODUCTIVE TECHNOLOGIES IN BUFFALO Giuseppina Maria Terzano Istituto Sperimentale per la Zootecnia (Animal Production Research Institute) Via Salaria 31, 00016 Monterotondo (Rome), Italy The general characteristics of reproduction like seasonality, cyclicity and ovulation differ widely in mammals for the following reasons: a) reproductive activity may take place during the whole year or at defined seasons, according to the species and their adaptation to environmental conditions; thus, photoperiod plays a determinant role in seasonal breeders such as rodents, carnivores and ruminants (sheep, goats, buffaloes, deer, etc.,). An extreme situation is observed in foxes with only one ovulation per year, occurring in January or February; b) mammals may be distinguished according to the absence or presence of spontaneous ovulations: in the first group of mammals ( rabbits, hares, cats, mink, camels, Llama), the ovulation is induced by mating and cyclicity is not obvious; in the second group, ovulation occurs spontaneously in each cycle, separating the follicular phase from the luteal phase; c) the length of cycles is quite different among species: small rodents have short cycles of four or five days, farm animals and primates have longer cycles (sheep: 17 days; cow, goat, buffalo, horse and pig: 21 days; primates: 28 days), and dogs are characterized by long cycles of six to seven months, including a two month luteal phase (Concannon, 1993); d) ovulation rates differ widely among species and breeds within a given species: in sheep for example, Merinos d'Arles or Ile-de-France breeds have only one ovulation per cycle, whereas average rates of two to four ovulations per cycle are observed in prolific breeds like Romanov or Finn (Land et al., 1973). -

Oogenesis in Mammals
OOGENESIS IN MAMMALS In contrast to most other vertebrates , mammals do not replenish the stores of oocytes present in the ovary at birth. At birth the human ovaries contain about 1 million oocytes ( many of which are already degenerating) that have been arrested in the diplotene stage of the first meiotic division . These oocytes are already surrounded by a layer of follicular cells or granulosa cells , and the complex of ovum and its surrounding cellular investments is known as a follicle . Of all the germ cells present in the ovary ,only about 400 (one per menstrual cycle) will reach maturity and become ovulated. The remainder develop to varying degrees and then undergo atresia (degeneration). Oocytes first become associated with follicular cells in the late fetal period , when they are going through early prophase of the first meiotic division . The primary oocyte (so called because it is undergoing the first meiotic division ) plus its incomplete covering of flattened follicular cells is called a primordial follicle . According to Gougeon (1993) a follicle passes through three major phases on its way to ovulation. The first phase is characterized by a large pool of nongrowing follicles , approximately 500,000 per ovary at birth. In this pool are primordial follicles, which develop into primary follicles by surrounding themselves with a complete single layer of cuboidal follicular cells . By this time , the oocytes have entered the first period of meiotic arrest , the diplotene stage . In human , essentially all oocytes , unless Page 1 of 5 : SEM-2 (GEN ) , Unit#6 : OOGENESIS : Pritha Mondal they degenerate ,remain arrested in the diplotene stage until puberty ; some will not progress past the diplotene stage until the woman’s last reproductive cycle (age 45 to 55 years). -

The Effectiveness and Safety of the Early Follicular Phase Full-Dose Down
The effectiveness and safety of the early follicular phase full-dose down- regulation protocol for controlled ovarian hyperstimulation: a randomized, paralleled controlled, multicenter trial 2018-12-29 Background Since the first “tube baby”, Louise Brown, was born in the United Kingdom in 1978, many infertile couples have been benefitted from in vitro fertilization and embryo transfer (IVF-ET) and intracytoplasmic sperm injection (ICSI). It is reported that there are over 5 million babies born with the help of assisted reproductive technology (ART). According to the 2015 national data published by Human Fertility and Embryology Authority (HFEA, 48,147 women received 61,726 IVF/ICSI cycles and gave birth to 17,041 newborns [1]. In the United States, 169,602 IVF/ICSI cycles were performed in 2014 and 68,791 tubal babies were born [2]. China has a huge population base, and therefore has a substantial number of infertile couples. Although a late starter, China is developing rapidly in ART and playing a more and more important role in the area of reproductive medicine. In spite of the continuous development in ART, so far, the overall success rate of IVF/ICSI is still hovering around 25-40%. The live birth rate per stimulated cycle is 25.6% in the UK in 2015, fluctuating from 1.9% in women aged 45 and elder to 32.2% in women younger than 35 years old [1]. The IVF/ICSI success rate in 2014 in the US is similar [2]. In China, according to the data submitted by 115 reproductive medicine centers on the ART data reporting system developed by Chinese Society of Reproductive Medicine, the delivery rate is about 40% [3]. -

Variability in the Length of Menstrual Cycles Within and Between Women - a Review of the Evidence Key Points
Variability in the Length of Menstrual Cycles Within and Between Women - A Review of the Evidence Key Points • Mean cycle length ranges from 27.3 to 30.1 days between ages 20 and 40 years, follicular phase length is 13-15 days, and luteal phase length is less variable and averages 13-14 days1-3 • Menstrual cycle lengths vary most widely just after menarche and just before menopause primarily as cycles are anovulatory 1 • Mean length of follicular phase declines with age3,11 while luteal phase remains constant to menopause8 • The variability in menstrual cycle length is attributable to follicular phase length1,11 Introduction Follicular and luteal phase lengths Menstrual cycles are the re-occurring physiological – variability of menstrual cycle changes that happen in women of reproductive age. Menstrual cycles are counted from the first day of attributable to follicular phase menstrual flow and last until the day before the next onset of menses. It is generally assumed that the menstrual cycle lasts for 28 days, and this assumption Key Points is typically applied when dating pregnancy. However, there is variability between and within women with regard to the length of the menstrual cycle throughout • Follicular phase length averages 1,11,12 life. A woman who experiences variations of less than 8 13-15 days days between her longest and shortest cycle is considered normal. Irregular cycles are generally • Luteal phase length averages defined as having 8 to 20 days variation in length of 13-14 days1-3 cycle, whereas over 21 days variation in total cycle length is considered very irregular. -
![Arxiv:1910.00443V3 [Eess.IV] 29 Jul 2020 Synchronize Acquired Data Sets, Such That Corresponding Developmental Stages Are Compared to One Another [3, 6]](https://docslib.b-cdn.net/cover/9873/arxiv-1910-00443v3-eess-iv-29-jul-2020-synchronize-acquired-data-sets-such-that-corresponding-developmental-stages-are-compared-to-one-another-3-6-709873.webp)
Arxiv:1910.00443V3 [Eess.IV] 29 Jul 2020 Synchronize Acquired Data Sets, Such That Corresponding Developmental Stages Are Compared to One Another [3, 6]
Towards Automatic Embryo Staging in 3D+t Microscopy Images using Convolutional Neural Networks and PointNets? Manuel Traub1;2[0000−0003−0897−1701] and Johannes Stegmaier1[0000−0003−4072−3759] 1 Institute of Imaging and Computer Vision, RWTH Aachen University, Germany 2 Institute for Automation and Applied Informatics, Karlsruhe Institute of Technology, Germany [email protected] Abstract. Automatic analyses and comparisons of different stages of embryonic development largely depend on a highly accurate spatiotem- poral alignment of the investigated data sets. In this contribution, we assess multiple approaches for automatic staging of developing embryos that were imaged with time-resolved 3D light-sheet microscopy. The methods comprise image-based convolutional neural networks as well as an approach based on the PointNet architecture that directly operates on 3D point clouds of detected cell nuclei centroids. The experiments with four wild-type zebrafish embryos render both approaches suitable for au- tomatic staging with average deviations of 21 − 34 minutes. Moreover, a proof-of-concept evaluation based on simulated 3D+t point cloud data sets shows that average deviations of less than 7 minutes are possible. Keywords: Convolutional Neural Networks · PointNet · Regression · Transfer Learning · Developmental Biology · Simulating Embryogenesis. 1 Introduction Embryonic development is characterized by a multitude of synchronized events, cell shape changes and large-scale tissue rearrangements that are crucial steps in the successful formation of a new organism [10]. To be able to compare these developmental events among different wild-type individuals, different mutants or upon exposure to certain chemicals, it is highly important to temporally arXiv:1910.00443v3 [eess.IV] 29 Jul 2020 synchronize acquired data sets, such that corresponding developmental stages are compared to one another [3, 6]. -

Effects of Caffeine, Alcohol and Smoking on Fertility
Pre-Conception Health Special Interest Group Effects of caffeine, alcohol and smoking on fertility There is an increasing body of evidence that health behaviours affect fertility. As most health behaviours can be modified, providing advice and support in making healthy changes can promote fertility. The evidence relating to the effects on fertility of caffeine, alcohol consumption and smoking is reviewed here. Your Fertility is a national public education campaign funded by the Australian Government Department of Health and Ageing under the Family Planning Grants Program. 1 Updated October 2015 Pre-Conception Health Special Interest Group Effects of caffeine, alcohol and smoking on fertility Evidence review Caffeine Smoking Caffeine is widely consumed as it is present in coffee, tea, some soft drinks There is strong evidence that smoking adversely affects male and female and chocolate. Some evidence suggests that the consumption of caffeine, fertility. Smokers are more likely to be infertile [7, 20-21] and women with a possible dose-response effect, may prolong the time to pregnancy who are exposed to smoking take longer to conceive [22]. Furthermore, and affect the health of a developing foetus, although the mechanism for maternal smoking increases the risk of low birth weight and birth defects this is unclear. Caffeine may affect ovulation and corpus luteum functioning [23] and women who smoke reach menopause earlier than non-smokers through alterations to hormone levels [1] and has been shown to be associated [24]. Smoking can also damage sperm DNA. Heavy smoking (≥20 with elevated early follicular E2 levels in females [2]. Although some studies cigarettes per day) by fathers at the time of conception increases the have found a positive relationship between caffeine consumption and time child’s risk of childhood leukaemia and shortens reproductive lifespan to conception [3-6], study results are inconsistent and should be interpreted of daughters [25-26]. -

Research Journal of Pharmaceutical, Biological and Chemical Sciences
ISSN: 0975-8585 Research Journal of Pharmaceutical, Biological and Chemical Sciences Alcohol Affects Pregnancy by Hypothalamic- Pituitary-Gonadal Axis Derangement. Nwangwa EK1*, Ekhoye EI1, Naiho AO1, and Ekene N2. 1Department of Physiology, 2Department of Pharmacology and Therapeutics Faculty of Basic Medical Sciences, College of Health Sciences, Delta State University, P.M.B. 001 Abraka. Delta State. Nigeria. ABSTRACT The present study investigated the deleterious effects of alcohol exposure on reproductive hormones during pregnancy. Forty Wistar albino rats (30 female & 10 male) were mated and randomized into 6 groups (n=5) and then administered with 25% alcohol daily through orogastric cannula from gestation day 0 to day 7 (Group 1), day 8 to day 14 (group 2) and day 15 to 21 (group 3). Group 4, 5 and 6 were control for 1, 2 and 3 respectively. At the end of each trimester, five rats were sacrificed from each experimental and corresponding control group and blood samples collected for FSH, LH, oestrogen and progesterone assay. The male rats were used for mating purposes only. One way analysis of Variance was used to compare means, p-value < 0.05 was considered significant. The result showed that alcohol decreased the FSH, LH, estrogen and progesterone levels during the course of the pregnancy with significance (p<0.05) especially in the third trimester. It was expected that there will be a rise in level of FSH and LH in response to decreasing oestrogen and progesterone level but that was not the case here This study therefore demonstrates that alcohol exposure adversely affects pregnancy through decrease in hormones that help in maintaining pregnancy especially in the third trimester possibly due to a derangement in the Hypothalamic- Pituitary-Gonadal (HPG) axis. -
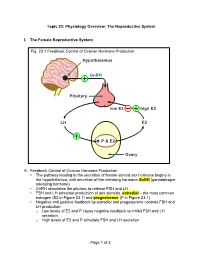
Of 3 Topic 23: Physiology Overview: the Reproductive System I. The
Topic 23: Physiology Overview: The Reproductive System I. The Female Reproductive System Fig. 23.1 Feedback Control of Ovarian Hormone Production Hypothalamus GnRH Pituitary low E2 high E2 LH E2 é P & E2 Ovary A. Feedback Control of Ovarian Hormone Production • The pathway leading to the secretion of female steroid sex hormone begins in the hypothalamus, with secretion of the releasing hormone GnRH (gonadotropin- releasing hormone) • GnRH stimulates the pituitary to release FSH and LH • FSH and LH stimulate production of sex steroids, estradiol – the most common estrogen (E2 in Figure 23.1) and progesterone (P in Figure 23.1) • Negative and positive feedback by estradiol and progesterone controls FSH and LH production: o Low levels of E2 and P cause negative feedback to inhibit FSH and LH secretion o High levels of E2 and P stimulate FSH and LH secretion Page 1 of 3 PCOL260 23. Physiology Overview: The Reproductive System B. The Menstrual Cycle Figure 23.2 The Menstrual Cycle Ovulation LH FSH Estradiol Relative Blood Level Progesterone 1287 14 21 Days of Menstrual Cycle 1. Follicular phase • The menstrual cycle begins as FSH stimulates development of an ovarian follicle (an egg surrounded by a covering of cells). As this follicle matures, it begins producing a small but steadily rising amount of estradiol. This low level of estradiol causes: o Negative feedback: inhibition of FSH and LH secretion o Growth of the lining of the uterus 2. Ovulation • The amount of estradiol secreted at mid-cycle has increased to the high level that stimulates a surge of FSH and LH release by the pituitary • The LH surge causes ovulation: the follicle ruptures and releases its egg near the opening of its fallopian tube 3. -

Twister Segment Morphological Filtering. a New Method for Live Zebrafish Embryos Confocal Images Processing
TWISTER SEGMENT MORPHOLOGICAL FILTERING. A NEW METHOD FOR LIVE ZEBRAFISH EMBRYOS CONFOCAL IMAGES PROCESSING. M.A.Luengo-Oroz§ , E.Faure‡, B.Lombardot‡, R.Sance§, P.Bourgine‡, N.Peyrieras´ and A. Santos§ §Biomedical Image Technologies lab., ETSI Telecomunicacion,´ Universidad Politecnica´ de Madrid, Spain ‡Centre de Recherche en Epistemologie´ Appliquee,´ CNRS - Ecole´ Polytechnique, Paris, France DEPSN, CNRS - Institut de Neurobiologie Alfred Fessard, Gif-sur-Yvette, France ABSTRACT which is an extension of a classical morphological filter, re- We propose an extension of the classical morphological filter- moves noise from live zebrafish embryo 4D images in a very ing based on openings by line segment structuring elements. efficient way, with a predictable computational cost. It consists in filtering a 3D+time image with the opening by all the possible rotations of a segment in the 4D space, giv- 2. TWISTER SEGMENT FILTERING en an initial segment and a rotation angle. The method has been applied to remove noise from confocal laser scanning 2.1. Preliminaries microscopy images of live zebrafish embryos engineered to A grey-tone image can be represented by a function f : fluorescently label all their cell membranes. 2 Df → T , where Df is a subset of Z and T = {tmin, ..., tmax} Index Terms— Biomedical image processing, image is an ordered set of grey-levels. Let B be a subset of Z2 and restoration, morphological operations, microscopy. s ∈ N a scaling factor. sB is called structuring element B of size s. The basic morphological operators are dilation [δ (f(x)) = sup {f(x − y)}] [ε (f(x)) = 1. INTRODUCTION B y∈B and erosion B ´ınf−y∈B{f(x − y)}]. -

Embryomics: Commercial Opportunities in the Increasingly Complex Biology of Pluripotency Case Western Reserve University
Embryomics: Commercial Opportunities in the Increasingly Complex Biology of Pluripotency Case Western Reserve University July 16, 2013 Forward Looking Statements The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability of BioTime and its licensees to obtain additional FDA and foreign regulatory approval to market BioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand for BioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTime and its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime's Securities and Exchange Commission filings. -

Impact of Transferring a Poor Quality Embryo Along with a Good Quality
Published online: 2020-08-14 GebFra Science | Original Article Impact of Transferring a Poor Quality Embryo Along with a Good Quality Embryo on Pregnancy Outcomes in IVF/ICSI Cycles: a Retrospective Study Auswirkungen des Transfers eines Embryos von schlechter Qualität zusammen mit einem Embryo von guter Qualität auf das Schwangerschafts-Outcome: eine retrospektive Studie Authors Oya Aldemir 1, Runa Ozelci 1,EmreBaser2, Iskender Kaplanoglu 1,SerdarDilbaz1, Berna Dilbaz 1, Ozlem Moraloglu Tekin 1 Affiliations ity embryo on the transfer day, double-embryo transfer (DET) 1 Department of Assisted Reproductive Technology, can be performed with these embryos, but generally, differ- Ankara Etlik Zubeyde HanımWomenʼs Health Training ent quality embryos are present in the available transfer co- and Research Hospital, Ankara, Turkey hort. We aimed to investigate the effect of transferring a poor 2 Department of Obstetrics and Gynaecology, quality embryo along with a good quality embryo on IVF out- Bozok University Medical Faculty, Yozgat, Turkey comes. Methods In this study, 2298 fresh IVF/intracytoplasmic Key words sperm injection (ICSI) cycles with two good quality embryos double embryo transfer, embryo quality, cleavage stage, (group A), one good and one poor quality embryo (group B), blastocyst stage, live birth rate, multiple pregnancy and single good quality embryo (group C) transfers were ex- amined. All groups were divided into two subgroups accord- Schlüsselwörter ing to the transfer day as cleavage or blastocyst stage. Clinical Doppelembryonentransfer, Embryoqualität, Teilungsstadium, pregnancy and live birth rates were the primary outcomes. Blastozystenstadium, Lebendgeburtenrate, Mehrlings- Results In the cleavage stage transfer subgroups, the clinical schwangerschaft pregnancy rates were lower in the single-embryo transfer (SET) subgroup compared with DET subgroups, but the differ- received 28.3.2020 ence was not statistically significant compared with DET with accepted after revision 7.7.2020 mixed quality embryos. -

Current Advancements in Noninvasive Profiling of the Embryo Culture
International Journal of Molecular Sciences Review Current Advancements in Noninvasive Profiling of the Embryo Culture Media Secretome Raminta Zmuidinaite 1 , Fady I. Sharara 2 and Ray K. Iles 1,3,* 1 MAP Sciences Ltd., The iLab, Stannard Way, Priory Business Park, Bedford MK44 3RZ, UK; [email protected] 2 Virginia Center for Reproductive Medicine, Reston, VA 20190, USA; [email protected] 3 NISAD (Lund), Medicon Village, SE-223 81 Lund, Sweden * Correspondence: [email protected] Abstract: There have been over 8 million babies born through in vitro fertilization (IVF) and this number continues to grow. There is a global trend to perform elective single embryo transfers, avoiding risks associated with multiple pregnancies. It is therefore important to understand where current research of noninvasive testing for embryos stands, and what are the most promising tech- niques currently used. Furthermore, it is important to identify the potential to translate research and development into clinically applicable methods that ultimately improve live birth and reduce time to pregnancy. The current focus in the field of human reproductive medicine is to develop a more rapid, quantitative, and noninvasive test. Some of the most promising fields of research for noninvasive as- says comprise cell-free DNA analysis, microscopy techniques coupled with artificial intelligence (AI) and omics analysis of the spent blastocyst media. High-throughput proteomics and metabolomics technologies are valuable tools for noninvasive embryo analysis. The biggest advantages of such technology are that it can differentiate between the embryos that appear morphologically identical and has the potential to identify the ploidy status noninvasively prior to transfer in a fresh cycle or Citation: Zmuidinaite, R.; Sharara, before vitrification for a later frozen embryo transfer.