Seven Genera, 15 Or More Species) Chile and New Zealand
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both. -

Chromosome Numbers in Compositae, XII: Heliantheae
SMITHSONIAN CONTRIBUTIONS TO BOTANY 0 NCTMBER 52 Chromosome Numbers in Compositae, XII: Heliantheae Harold Robinson, A. Michael Powell, Robert M. King, andJames F. Weedin SMITHSONIAN INSTITUTION PRESS City of Washington 1981 ABSTRACT Robinson, Harold, A. Michael Powell, Robert M. King, and James F. Weedin. Chromosome Numbers in Compositae, XII: Heliantheae. Smithsonian Contri- butions to Botany, number 52, 28 pages, 3 tables, 1981.-Chromosome reports are provided for 145 populations, including first reports for 33 species and three genera, Garcilassa, Riencourtia, and Helianthopsis. Chromosome numbers are arranged according to Robinson’s recently broadened concept of the Heliantheae, with citations for 212 of the ca. 265 genera and 32 of the 35 subtribes. Diverse elements, including the Ambrosieae, typical Heliantheae, most Helenieae, the Tegeteae, and genera such as Arnica from the Senecioneae, are seen to share a specialized cytological history involving polyploid ancestry. The authors disagree with one another regarding the point at which such polyploidy occurred and on whether subtribes lacking higher numbers, such as the Galinsoginae, share the polyploid ancestry. Numerous examples of aneuploid decrease, secondary polyploidy, and some secondary aneuploid decreases are cited. The Marshalliinae are considered remote from other subtribes and close to the Inuleae. Evidence from related tribes favors an ultimate base of X = 10 for the Heliantheae and at least the subfamily As teroideae. OFFICIALPUBLICATION DATE is handstamped in a limited number of initial copies and is recorded in the Institution’s annual report, Smithsonian Year. SERIESCOVER DESIGN: Leaf clearing from the katsura tree Cercidiphyllumjaponicum Siebold and Zuccarini. Library of Congress Cataloging in Publication Data Main entry under title: Chromosome numbers in Compositae, XII. -
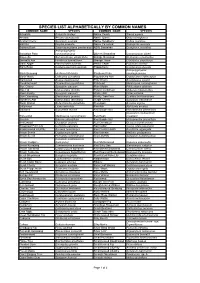
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Plant Life of Western Australia
INTRODUCTION The characteristic features of the vegetation of Australia I. General Physiography At present the animals and plants of Australia are isolated from the rest of the world, except by way of the Torres Straits to New Guinea and southeast Asia. Even here adverse climatic conditions restrict or make it impossible for migration. Over a long period this isolation has meant that even what was common to the floras of the southern Asiatic Archipelago and Australia has become restricted to small areas. This resulted in an ever increasing divergence. As a consequence, Australia is a true island continent, with its own peculiar flora and fauna. As in southern Africa, Australia is largely an extensive plateau, although at a lower elevation. As in Africa too, the plateau increases gradually in height towards the east, culminating in a high ridge from which the land then drops steeply to a narrow coastal plain crossed by short rivers. On the west coast the plateau is only 00-00 m in height but there is usually an abrupt descent to the narrow coastal region. The plateau drops towards the center, and the major rivers flow into this depression. Fed from the high eastern margin of the plateau, these rivers run through low rainfall areas to the sea. While the tropical northern region is characterized by a wet summer and dry win- ter, the actual amount of rain is determined by additional factors. On the mountainous east coast the rainfall is high, while it diminishes with surprising rapidity towards the interior. Thus in New South Wales, the yearly rainfall at the edge of the plateau and the adjacent coast often reaches over 100 cm. -

SIPARUNACEAE E MONIMIACEAE Siparuna Gêneros Pelos Reserva Na Representadas Estão Semelhantes
146 Siparunaceae e Monimiaceae As duas famílias foram recentemente monóicas ou dióicas. Os frutos são encobertos separadas. Monimiaceae sempre foi pelo receptáculo carnoso e quando maduros considerada heterogênea, e estudos atuais rompem-se irregularmente (com exceção de S. apontam para a segregação de certos gêneros glycycarpa, que é indeiscente), expondo drupas no nível de família, como o gênero Siparuna semelhantes a sementes, com arilo vermelho para Siparunaceae. São tratadas juntas neste ou alaranjado rico em lipídios. guia por serem vegetativamente semelhantes. As flores de Siparuna são polinizadas por Estão representadas na Reserva pelos gêneros moscas de hábito noturno, que visitam as flores Siparuna e Mollinedia. para acasalamento e oviposição. Monimiaceae é predominantemente pantropical com aproximadamente 194 espécies em ca. 22 gêneros, com a mesma distribuição de Siparunaceae na região neotropical. Na Amazônia ocorre somente o gênero Mollinedia, com uma espécie dióica, M. ovata registrada na Reserva. No campo a família é identificada através das mesmas características usadas para Siparunaceae. Mollinedia ovata tem folhas subcoriáceas, venação broquidódroma e poucas secundárias. As flores são como em Siparuna guianensis Siparunaceae, o receptáculo apresentando a Siparunaceae é neotropical e contém margem sinuosa. Os frutos são drupas sésseis aproximadamente 70 espécies em 2 gêneros ou estipitadas (semelhantes aos de Guatteria, (apenas um gênero mono-específico na Annonaceae) ou como em Siparunaceae, mas África Ocidental, Glossocalyx), distribuídas raramente com arilo alaranjado. desde o México e o Caribe até o Paraguai e Argentina. Somente Siparuna ocorre na Amazônia brasileira, estando representada na Reserva por nove espécies de hábito arbóreo ou arbustivo. O reconhecimento da família no campo é fácil: a lâmina foliar tem pontuações translúcidas e a planta tem um odor cítrico, agradável ou fétido (de onde vem o nome indígena caá-pitiú, ou planta com cheiro ruim), devido à grande concentração de óleos essenciais. -

Presencia De Fumagina Por Altitud Y Ecosistemas
ESCUELA SUPERIOR POLITÉCNICA DE CHIMBORAZO FACULTAD DE RECURSOS NATURALES ESCUELA DE INGENIERÍA EN ECOTURISMO CONSERVACIÓN DE Loricaria illinissae A TRAVÉS DEL ESTUDIO DE LA ENTOMOFAUNA ASOCIADA, EN LA RESERVA DE PRODUCCIÓN DE FAUNA CHIMBORAZO TRABAJO DE TITULACIÓN PROYECTO DE INVESTIGACIÓN PARA TITULACIÓN DE GRADO PRESENTADO COMO REQUISITO PARCIAL PARA OBTENER EL TÍTULO DE INGENIERO EN ECOTURISMO PATRICIA NATALIA CRUZ ROMÁN RIOBAMBA-ECUADOR 2018 ii ©2018, Patricia Natalia Cruz Román Se autoriza la reproducción total o parcial, con fines académicos, por cualquier medio o procedimiento, incluyendo la cita bibliográfica del documento, siempre y cuando se reconozca el Derecho de Autor iii iv v DEDICATORIA Con mucho cariño quiero dedicar este trabajo a mi madre, que ha estado junto a mí en todo momento, con su apoyo incondicional y esfuerzo diario para ayudarme a alcanzar esta meta. vi AGRADECIMIENTO A mi Familia, de forma particular a mi madre, hermano, y esposo por su apoyo moral y económico durante todo este proceso. A la Escuela Superior Politécnica de Chimborazo, que me ha abierto sus puertas y es la institución que hoy me permite cumplir esta meta. A todos quienes conforman el equipo del MAE, por los conocimientos compartidos y sobre todo por la calidad humana de este gran grupo de profesionales. A los ingenieros: Armando Espinoza y Juan Carlos Carrasco; por la guía constante durante este proceso. vii Tabla de contenido CONSERVACIÓN DE Loricaria illinissae A TRAVÉS DEL ESTUDIO DE LA ENTOMOFAUNA ASOCIADA, EN LA RESERVA DE PRODUCCIÓN -

Reconstructing the Basal Angiosperm Phylogeny: Evaluating Information Content of Mitochondrial Genes
55 (4) • November 2006: 837–856 Qiu & al. • Basal angiosperm phylogeny Reconstructing the basal angiosperm phylogeny: evaluating information content of mitochondrial genes Yin-Long Qiu1, Libo Li, Tory A. Hendry, Ruiqi Li, David W. Taylor, Michael J. Issa, Alexander J. Ronen, Mona L. Vekaria & Adam M. White 1Department of Ecology & Evolutionary Biology, The University Herbarium, University of Michigan, Ann Arbor, Michigan 48109-1048, U.S.A. [email protected] (author for correspondence). Three mitochondrial (atp1, matR, nad5), four chloroplast (atpB, matK, rbcL, rpoC2), and one nuclear (18S) genes from 162 seed plants, representing all major lineages of gymnosperms and angiosperms, were analyzed together in a supermatrix or in various partitions using likelihood and parsimony methods. The results show that Amborella + Nymphaeales together constitute the first diverging lineage of angiosperms, and that the topology of Amborella alone being sister to all other angiosperms likely represents a local long branch attrac- tion artifact. The monophyly of magnoliids, as well as sister relationships between Magnoliales and Laurales, and between Canellales and Piperales, are all strongly supported. The sister relationship to eudicots of Ceratophyllum is not strongly supported by this study; instead a placement of the genus with Chloranthaceae receives moderate support in the mitochondrial gene analyses. Relationships among magnoliids, monocots, and eudicots remain unresolved. Direct comparisons of analytic results from several data partitions with or without RNA editing sites show that in multigene analyses, RNA editing has no effect on well supported rela- tionships, but minor effect on weakly supported ones. Finally, comparisons of results from separate analyses of mitochondrial and chloroplast genes demonstrate that mitochondrial genes, with overall slower rates of sub- stitution than chloroplast genes, are informative phylogenetic markers, and are particularly suitable for resolv- ing deep relationships. -

Phylogeny and Evolution of Achenial Trichomes In
Luebert & al. • Achenial trichomes in the Lucilia-group (Asteraceae) TAXON 66 (5) • October 2017: 1184–1199 Phylogeny and evolution of achenial trichomes in the Lucilia-group (Asteraceae: Gnaphalieae) and their systematic significance Federico Luebert,1,2,3 Andrés Moreira-Muñoz,4 Katharina Wilke2 & Michael O. Dillon5 1 Freie Universität Berlin, Institut für Biologie, Botanik, Altensteinstraße 6, 14195 Berlin, Germany 2 Universität Bonn, Nees-Institut für Biodiversität der Pflanzen, Meckenheimer Allee 170, 53115 Bonn, Germany 3 Universidad de Chile, Departamento de Silvicultura y Conservación de la Naturaleza, Santiago, Chile 4 Pontificia Universidad Católica de Valparaíso, Instituto de Geografía, Avenida Brasil 2241, Valparaíso, Chile 5 The Field Museum, Integrative Research Center, 1400 South Lake Shore Drive, Chicago, Illinois 60605, U.S.A. Author for correspondence: Federico Luebert, [email protected] ORCID FL, http://orcid.org/0000000322514056; MOD, http://orcid.org/0000000275120766 DOI https://doi.org/10.12705/665.11 Abstract The Gnaphalieae (Asteraceae) are a cosmopolitan tribe with around 185 genera and 2000 species. The New World is one of the centers of diversity of the tribe with 24 genera and over 100 species, most of which form a clade called the Luciliagroup with 21 genera. However, the generic classification of the Luciliagroup has been controversial with no agreement on delimitation or circumscription of genera. Especially controversial has been the taxonomic value of achenial trichomes and molecular studies have shown equivocal results so far. The major aims of this paper are to provide a nearly complete phylogeny of the Lucilia group at generic level and to discuss the evolutionary trends and taxonomic significance of achenial trichome morphology. -

Tasmania - from the Wet West to the Dry East
This collection is maintained with the assistance of the Tasmania - from the wet west to the dry east. Regional Branch of the Australian Plant Society. Influences on the development of the Tasmanian plant mix Montane moorland and cool oceanic heathland When Gondwana existed as a super Geology of Tasmania Vegetation Map of Tasmania The Tasmanian highland vegetation developed in isolation from the Australian Alps. Even during ice continent, Australia and Tasmania, Africa, ages, hundreds of kilometres of lowland vegetation separated the two high altitude environments. South America, New Zealand and Antarctica shared many plant families and some Montane plants have to cope with wide temperature fluctuations, with periods of below 0°C and Genera. exposure to winds. Cold may be prolonged if the ground freezes. Plants may be blanketed by snow or BASS STRAIT the mountains by cloud. Snowmelt or clear weather can cause intense rays of light, resulting in high For example, the protea family has members temperature. Wind or sun can dry the plant and soil. in all those land masses except Antarctica. The Southern Africa panel covers the protea family more fully. Plants require moisture and warmth. Small hard leaves offer Tasmania was the last land mass to break protection from the drying away from Antarctica. The opening of the effects of sun and wind. Low gap between these land masses allowed the ocean to circulate growth avoids wind. Branches around Antarctica, cooling the earth’s climate and so locking up grow close together to shelter vast quantities of water as ice. the parts of each plant. -

Fauna Survey, Wingham Management Area, Port Macquarie Region
This document has been scanned from hard-copy archives for research and study purposes. Please note not all information may be current. We have tried, in preparing this copy, to make the content accessible to the widest possible audience but in some cases we recognise that the automatic text recognition maybe inadequate and we apologise in advance for any inconvenience this may cause. FOREST RESOURCES SERIES NO. 19 FAUNA SURVEY, WINGHAM MANAGEMENT AREA, PORT MACQUARIE REGION PART 1. MAMMALS BY ALAN YORK \ \ FORESTRY COMMISSION OF NEW SOUTH WALES ----------------------------- FAUNA SURVEY, WINGHAM MANAGEMENT AREA, PORT MACQUARIE REGION PART 1. MAMMALS by ALAN YORK FOREST ECOLOGY SECTION WOOD TECHNOLOGY AND FOREST RESEARCH DIVISION FORESTRY COMMISSION OF NEW SOUTH WALES SYDNEY 1992 Forest Resources Series No. 19 March 1992 The Author: AIan York, BSc.(Hons.) PhD., Wildlife Ecologist, Forest Ecology Section, Wood Technology and Forest Research Division, Forestry Commission ofNew South Wales. Published by: Forestry Commission ofNew South Wales, Wood Technology and Forest Research Division, 27 Oratava Avenue, West Pennant Hills, 2125 P.O. Box lOO, Beecroft 2119 Australia. Copyright © 1992 by Forestry Commission ofNew South Wales ODC 156.2:149 (944) ISSN 1033-1220 ISBN 07305 5663 8 Fauna Survey, Wingham Management Area, -i- PortMacquarie Region Part 1. Mammals TABLE OF CONTENTS PAGE INTRODUCTION 1 1. The Wingham Management Area 1 (a) Location 1 (b) Physical environment 3 (c) Vegetation communities 3 (d) Fire 5 (e) Timber harvesting : 5 SURVEY METHODOLOGy 7 1. Overall Sampling Strategy 7 (a) General survey 7 (b) Plot-based survey 7 (i) Stratification 1:Jy Broad Forest Type : 8 (ii) Stratification by Altitude 8 (iii) Stratification 1:Jy Management History 8 (iv) Plot selection 9 (v) Special considerations 9 (vi) Plot establishment 10 (vii) Plot design........................................................................................................... -

(OUV) of the Wet Tropics of Queensland World Heritage Area
Handout 2 Natural Heritage Criteria and the Attributes of Outstanding Universal Value (OUV) of the Wet Tropics of Queensland World Heritage Area The notes that follow were derived by deconstructing the original 1988 nomination document to identify the specific themes and attributes which have been recognised as contributing to the Outstanding Universal Value of the Wet Tropics. The notes also provide brief statements of justification for the specific examples provided in the nomination documentation. Steve Goosem, December 2012 Natural Heritage Criteria: (1) Outstanding examples representing the major stages in the earth’s evolutionary history Values: refers to the surviving taxa that are representative of eight ‘stages’ in the evolutionary history of the earth. Relict species and lineages are the elements of this World Heritage value. Attribute of OUV (a) The Age of the Pteridophytes Significance One of the most significant evolutionary events on this planet was the adaptation in the Palaeozoic Era of plants to life on the land. The earliest known (plant) forms were from the Silurian Period more than 400 million years ago. These were spore-producing plants which reached their greatest development 100 million years later during the Carboniferous Period. This stage of the earth’s evolutionary history, involving the proliferation of club mosses (lycopods) and ferns is commonly described as the Age of the Pteridophytes. The range of primitive relict genera representative of the major and most ancient evolutionary groups of pteridophytes occurring in the Wet Tropics is equalled only in the more extensive New Guinea rainforests that were once continuous with those of the listed area. -

Phylogeny, Molecular Dating, and Floral Evolution of Magnoliidae (Angiospermae)
UNIVERSITÉ PARIS-SUD ÉCOLE DOCTORALE : SCIENCES DU VÉGÉTAL Laboratoire Ecologie, Systématique et Evolution DISCIPLINE : BIOLOGIE THÈSE DE DOCTORAT Soutenue le 11/04/2014 par Julien MASSONI Phylogeny, molecular dating, and floral evolution of Magnoliidae (Angiospermae) Composition du jury : Directeur de thèse : Hervé SAUQUET Maître de Conférences (Université Paris-Sud) Rapporteurs : Susanna MAGALLÓN Professeur (Universidad Nacional Autónoma de México) Thomas HAEVERMANS Maître de Conférences (Muséum national d’Histoire Naturelle) Examinateurs : Catherine DAMERVAL Directeur de Recherche (CNRS, INRA) Michel LAURIN Directeur de Recherche (CNRS, Muséum national d’Histoire Naturelle) Florian JABBOUR Maître de Conférences (Muséum national d’Histoire Naturelle) Michael PIRIE Maître de Conférences (Johannes Gutenberg Universität Mainz) Membres invités : Hervé SAUQUET Maître de Conférences (Université Paris-Sud) Remerciements Je tiens tout particulièrement à remercier mon directeur de thèse et ami Hervé Sauquet pour son encadrement, sa gentillesse, sa franchise et la confiance qu’il m’a accordée. Cette relation a immanquablement contribuée à ma progression humaine et scientifique. La pratique d’une science sans frontière est la plus belle chose qu’il m’ait apportée. Ce fut enthousiasmant, très fructueux, et au-delà de mes espérances. Ce mode de travail sera le mien pour la suite de ma carrière. Je tiens également à remercier ma copine Anne-Louise dont le soutien immense a contribué à la réalisation de ce travail. Elle a vécu avec patience et attention les moments d’enthousiasmes et de doutes. Par la même occasion, je remercie ma fille qui a eu l’heureuse idée de ne pas naître avant la fin de la rédaction de ce manuscrit.