Important Information About Vaccinia (Smallpox) Vaccine Please Read This Carefully
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Smallpox ( Variola )
Division of Disease Control What Do I Need to Know? Smallpox ( Variola ) What is Smallpox? Smallpox is a serious contagious disease that can be fatal. It is caused by the variola virus, a member of the Poxviridae family. The World Health Organization declared smallpox eliminated from the world in 1980. Who is at risk for Smallpox? Because smallpox has been eliminated, no one will be exposed to a naturally occurring case of smallpox. Smallpox is considered one of the viruses that maybe used in an attack by bioterrorists. Anyone who has not received smallpox vaccine is at risk if exposed to the virus. Also, people who received the vaccine may be at risk if exposed to smallpox because the protection declines five to ten years after one dose and possibly longer after two or more doses. The United States stopped routine childhood vaccination against smallpox in 1972. The last case of smallpox in the United States was in 1949 and the last naturally occurring case occurred in 1977 in Somalia. What are the symptoms of Smallpox? The first stage of the illness lasts two to five days with symptoms that include a high fever of 102˚F – 104˚F, a general feeling of discomfort, severe headache, backache, abdominal pain, and weakness. Most people will be severely ill and bedridden during this stage. By the third or fourth day of this stage, the fever usually drops. Following this stage, soars appear in the mouth and throat, but they may not be noticed by the infected person. The rash begins less than 24 hours after the sores in the mouth and throat appear. -

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -

Vaccinia Belongs to a Family of Viruses That Is Closely Related to the Smallpox Virus
VACCINIA INFECTION What is it? Vaccinia belongs to a family of viruses that is closely related to the smallpox virus. Because of the similarities between the smallpox and vaccinia viruses, the vaccinia virus is used in the smallpox vaccine. When this virus is used as a vaccine, it allows our immune systems to develop immunity against smallpox. The smallpox vaccine does not actually contain smallpox virus and cannot cause smallpox. Vaccination usually prevents smallpox infection for at least ten years. The vaccinia vaccine against smallpox was used to successfully eradicate smallpox from the human population. More recently, this virus has also become of interest due to concerns about smallpox being used as an agent of bioterrorism. How is the virus spread? Vaccinia can be spread by touching the vaccination site before it has fully healed or by touching clothing or bandages that have been contaminated with the live virus during vaccination. In this manner, vaccinia can spread to other parts of the body and to other individuals. It cannot be spread through the air. What are the symptoms of vaccinia? Vaccinia virus symptoms are similar to smallpox, but milder. Vaccinia may cause rash, fever, headache and body aches. In certain individuals, such as those with weak immune systems, the symptoms can be more severe. What are the potential side effects of the vaccinia vaccine for smallpox? Normal reactions are mild and go away without any treatment.These include: Soreness and redness in the arm where the vaccine was given Slightly swollen, sore glands in the armpits Low grade fever One in approximately three people will feel badly enough to miss school, work or recreational activities Trouble sleeping Serious reactions are not very common but can occur in about 1,000 in every 1 million people who are vaccinated for the first time. -

Vaccines and Autism: What You Should Know | Vaccine Education
Q A Vaccines and Autism: What you should know Volume& 1 Summer 2008 Some parents of children with autism are concerned that vaccines are the cause. Their concerns center on three areas: the combination measles-mumps-rubella (MMR) vaccine; thimerosal, a mercury-containing preservative previously contained in several vaccines; and the notion that babies receive too many vaccines too soon. Q. What are the symptoms of autism? Q. Does the MMR vaccine cause autism? A. Symptoms of autism, which typically appear during the A. No. In 1998, a British researcher named Andrew Wakefi eld fi rst few years of life, include diffi culties with behavior, social raised the notion that the MMR vaccine might cause autism. skills and communication. Specifi cally, children with autism In the medical journal The Lancet, he reported the stories of may have diffi culty interacting socially with parents, siblings eight children who developed autism and intestinal problems and other people; have diffi culty with transitions and need soon after receiving the MMR vaccine. To determine whether routine; engage in repetitive behaviors such as hand fl apping Wakefi eld’s suspicion was correct, researchers performed or rocking; display a preoccupation with activities or toys; a series of studies comparing hundreds of thousands of and suffer a heightened sensitivity to noise and sounds. children who had received the MMR vaccine with hundreds Autism spectrum disorders vary in the type and severity of of thousands who had never received the vaccine. They found the symptoms they cause, so two children with autism may that the risk of autism was the same in both groups. -

The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats
Acta Derm Venereol 2012; 92: 126–131 INVESTIGATIVE REPORT The Munich Outbreak of Cutaneous Cowpox Infection: Transmission by Infected Pet Rats Sandra VOGEL1, Miklós SÁRDY1, Katharina GLOS2, Hans Christian KOrting1, Thomas RUZICKA1 and Andreas WOLLENBERG1 1Department of Dermatology and Allergology, Ludwig Maximilian University, Munich, and 2Department of Dermatology, Haas and Link Animal Clinic, Germering, Germany Cowpox virus infection of humans is an uncommon, another type of orthopoxvirus, from infected pet prairie potentially fatal, skin disease. It is largely confined to dogs have recently been described in the USA, making Europe, but is not found in Eire, or in the USA, Austral the medical community aware of the risk of transmission asia, or the Middle or Far East. Patients having contact of pox viruses from pets (3). with infected cows, cats, or small rodents sporadically Seven of 8 exposed patients living in the Munich contract the disease from these animals. We report here area contracted cowpox virus infection from an unusual clinical aspects of 8 patients from the Munich area who source: rats infected with cowpox virus bought from had purchased infected pet rats from a local supplier. Pet local pet shops and reputedly from the same supplier rats are a novel potential source of local outbreaks. The caused a clinically distinctive pattern of infection, which morphologically distinctive skin lesions are mostly res was mostly restricted to the patients’ neck and trunk. tricted to the patients’ necks, reflecting the infected ani We report here dermatologically relevant aspects of mals’ contact pattern. Individual lesions vaguely resem our patients in order to alert the medical community to ble orf or Milker’s nodule, but show marked surrounding the possible risk of a zoonotic orthopoxvirus outbreak erythema, firm induration and local adenopathy. -

Smallpox Overview
SMALLPOX FACT SHEET Smallpox Overview The Disease Smallpox is a serious, contagious, and sometimes fatal infectious disease. There is no specific treatment for smallpox disease, and the only prevention is vaccination. The name smallpox is derived from the Latin word for “spotted” and refers to the raised bumps that appear on the face and body of an infected person. There are two clinical forms of smallpox. Variola major is the severe and most common form of smallpox, with a more extensive rash and higher fever. There are four types of variola major smallpox: ordinary (the most frequent type, accounting for 90% or more of cases); modified (mild and occurring in previously vaccinated persons); flat; and hemorrhagic (both rare and very severe). Historically, variola major has an overall fatality rate of about 30%; however, flat and hemorrhagic smallpox usually are fatal. Variola minor is a less common presentation of smallpox, and a much less severe disease, with death rates historically of 1% or less. Smallpox outbreaks have occurred from time to time for thousands of years, but the disease is now eradicated after a successful worldwide vaccination program. The last case of smallpox in the United States was in 1949. The last naturally occurring case in the world was in Somalia in 1977. After the disease was eliminated from the world, routine vaccination against smallpox among the general public was stopped because it was no longer necessary for prevention. Where Smallpox Comes From Smallpox is caused by the variola virus that emerged in human populations thousands of years ago. Except for laboratory stockpiles, the variola virus has been eliminated. -
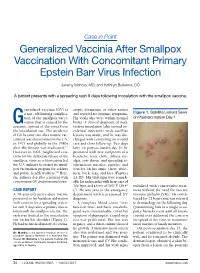
Generalized Vaccinia After Smallpox Vaccination with Concomitant Primary Epstein Barr Virus Infection
Case in Point Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection Jeremy Mandia, MD; and Kathryn Buikema, DO A patient presents with a spreading rash 9 days following inoculation with the smallpox vaccine. eneralized vaccinia (GV) is atopic dermatitis, or other rashes a rare, self-limiting complica- and reported no systemic symptoms. Figure 1. Satellite Lesions Seen tion of the smallpox vacci- His vitals also were within normal on Postvaccination Day 1 Gnation that is caused by the limits. A clinical diagnosis of inad- systemic spread of the virus from vertent inoculation (also termed ac- the inoculation site. The incidence cidental infection) with satellite of GV became rare after routine vac- lesions was made, and he was dis- cination was discontinued in the U.S. charged with counseling on wound in 1971 and globally in the 1980s care and close follow-up. Two days after the disease was eradicated.1,2 later, on postvaccination day 11, he However in 2002, heightened con- presented with new symptoms of a cerns for the deliberate release of the headache, fever, chills, diffuse my- smallpox virus as a bioweapon led algia, sore throat, and spreading er- the U.S. military to restart its small- ythematous macules, papules, and pox vaccination program for soldiers vesicles on his arms, chest, abdo- and public health workers.3,4 Here, men, back, legs, and face (Figures the authors describe a patient with 2A-2D). His vital signs were remark- concomitant GV and mononucleosis. able for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º stabilized with conservative treat- CASE REPORT C). -

A Guide to Vaccinations for Parents
A GUIDE TO VACCINATIONS FOR PARENTS What are vaccines? When should my child be vaccinated? Why does my child need the HPV vaccine? HISTORY OF VACCINATIONS Smallpox is a serious infectious disease that causes fever and a distinctive, progressive 600 1796 skin rash. years ago Edward Jenner developed Variolation, intentionally a vaccine against smallpox. Cases of paralysis from polio in the U.S. exposing an individual to Almost 200 years later, in 1980, in the early 1950s: smallpox material, traces back the World Health Organization more than to 16th-century China. This declared that smallpox process resulted in a milder had been eradicated, 15,000 form of the disease. or wiped out. In the year 2017: Childhood vaccines can prevent 14 potentially serious 0 diseases or conditions throughout your child’s lifetime. 1955 1940s 1885 Jonas Salk’s polio vaccine The routine immunization Louis Pasteur developed a was proven safe and effective. schedule included vaccines vaccine against rabies. The Polio has now been eliminated against four potentially serious rabies vaccine series, which in the U.S., and organizations diseases (smallpox, diphtheria, can be given to people who are currently working tetanus, and pertussis). may have been exposed to to eradicate polio Now the schedule includes the virus, has made rabies worldwide. vaccines to prevent a total infection very rare in the of 14 conditions. United States. In the U.S., vaccines go through three phases of clinical trials to make sure they are safe and effective before they are licensed. 2006 Today The HPV vaccine was Vaccine research licensed in the U.S. -

Vaccinia Viruses As Vectors for Vaccine Antigens Vaccinia Viruses As Vectors for Vaccine Antigens
Vaccinia Viruses as Vectors for Vaccine Antigens Vaccinia Viruses as Vectors for Vaccine Antigens Proceedings of the Workshop on Vaccinia Viruses as Vectors for Vaccine Antigens, held November 13-14, 1984, in Chevy Chase, Maryland, U. S.A. Editor: GeraldV.Quinnan,Jr.,M.D. Director, Division of Virology Center for Drugs and Biologics Food and Drug Administration Bethesda, Maryland Elsevier New York • Amsterdam. Oxford .... i © 1985 by Elsevier Science Publishing Co., Inc. All rights reserved. This book has been registered with the Copyright Clearance Center, Inc. For further information, please contact the Copyright Clearance Center, Salem, Massachusetts. Published by: Elsevier Science Publishing Co., Inc. 52 Vanderbilt Avenue, New York, New York 10017 Sole distributors outside the United States and Canada: Elsevier Science Publishers B.V. P.O. Box 211, 1000 AE Amsterdam, the Netherlands Library of Congress Cataloging in Publication Data Workshop in Vaccinia Viruses as Vectors for Vaccine Antigens (1984: Chevy Chase, Md.) Vaccinia viruses as vectors for vaccine antigens. Includes index. I. Vaccines--Congresses. 2. Vaccinia--Congresses. 3. Viralantigens-- Congresses. 4. Smallpox--Congresses. I. Quinnan, Gerald V. 1I. Title. [DNLM: 1. Antigens, Viral--immunology--Congresses. 2. VacciniaVirus-- genetics--congresses. 3. VacciniaVirus--immunology--congresses. 4. Viral Vaccines--immunology--congresses. QW 165.5.P6 W926v 1984] QR189.W674 1984 615'.372 85-12960 ISBN 0-444-00984-1 Manufactured in the United States of America CONTENTS Preface ..... ..... ° . ............... ix Participants .............................. xi Part I: BIOLOGY OF VACCINIA AND OTHER ORTHOPOX VIRUSES I Chairpersons: G. Schild and J. Nakano Vaccinia Virus ...................... 3 D. Baxby Aspects of the Biology of Orthopox Viruses Relevant to the Use of Recombinant Vaccines as Field Vaccines ..... -

Medical Management of Biological Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES FORT DETRICK FREDERICK, MARYLAND Emergency Response Numbers National Response Center: 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Helpline: 1-410-436-4484 or (Edgewood Ops Center – for military use) DSN 584-4484 USAMRIID’s Emergency Response Line: 1-888-872-7443 CDC'S Emergency Response Line: 1-770-488-7100 Handbook Download Site An Adobe Acrobat Reader (pdf file) version of this handbook can be downloaded from the internet at the following url: http://www.usamriid.army.mil USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 Lead Editor Lt Col Jon B. Woods, MC, USAF Contributing Editors CAPT Robert G. Darling, MC, USN LTC Zygmunt F. Dembek, MS, USAR Lt Col Bridget K. Carr, MSC, USAF COL Ted J. Cieslak, MC, USA LCDR James V. Lawler, MC, USN MAJ Anthony C. Littrell, MC, USA LTC Mark G. Kortepeter, MC, USA LTC Nelson W. Rebert, MS, USA LTC Scott A. Stanek, MC, USA COL James W. Martin, MC, USA Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 PREFACE TO THE SIXTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

Specimen Type, Collection Methods, and Diagnostic Assays Available For
Specimen type, collection methods, and diagnostic assays available for the detection of poxviruses from human specimens by the Poxvirus and Rabies Branch, Centers for Disease Control and Prevention1. Specimen Orthopoxvirus Parapoxvirus Yatapoxvirus Molluscipoxvirus Specimen type collection method PCR6 Culture EM8 IHC9,10 Serology11 PCR12 EM8 IHC9,10 PCR13 EM8 PCR EM8 Lesion material Fresh or frozen Swab 5 Lesion material [dry or in media ] [vesicle / pustule Formalin fixed skin, scab / crust, etc.] Paraffin block Fixed slide(s) Container Lesion fluid Swab [vesicle / pustule [dry or in media5] fluid, etc.] Touch prep slide Blood EDTA2 EDTA tube 7 Spun or aliquoted Serum before shipment Spun or aliquoted Plasma before shipment CSF3,4 Sterile 1. The detection of poxviruses by electron microscopy (EM) and immunohistochemical staining (IHC) is performed by the Infectious Disease Pathology Branch of the CDC. 2. EDTA — Ethylenediaminetetraacetic acid. 3. CSF — Cerebrospinal fluid. 4. In order to accurately interpret test results generated from CSF specimens, paired serum must also be submitted. 5. If media is used to store and transport specimens a minimal amount should be used to ensure as little dilution of DNA as possible. 6. Orthopoxvirus generic real-time polymerase chain reaction (PCR) assays will amplify DNA from numerous species of virus within the Orthopoxvirus genus. Species-specific real-time PCR assays are available for selective detection of DNA from variola virus, vaccinia virus, monkeypox virus, and cowpox virus. 7. Blood is not ideal for the detection of orthopoxviruses by PCR as the period of viremia has often passed before sampling occurs. 8. EM can reveal the presence of a poxvirus in clinical specimens or from virus culture, but this technique cannot differentiate between virus species within the same genus.