Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I4X-JE-JFCM an Open-Label, Multicenter, Phase 1B/2 Study To
Protocol (e) I4X-JE-JFCM An Open-label, Multicenter, Phase 1b/2 Study to Evaluate Necitumumab in Combination with Gemcitabine and Cisplatin in the First-Line Treatment of Patients with Advanced (Stage IV) Squamous Non-Small Cell Lung Cancer (NSCLC) NCT01763788 Approval Date: 12-Jun-2016 I4X-JE-JFCM(e) Clinical Protocol Page 1 1. Protocol I4X-JE-JFCM(e) An Open-label, Multicenter, Phase 1b/2 Study to Evaluate Necitumumab in Combination with Gemcitabine and Cisplatin in the First-Line Treatment of Patients with Advanced (Stage IV) Squamous Non-Small Cell Lung Cancer (NSCLC) Confidential Information The information contained in this protocol is confidential and is intended for the use of clinical investigators. It is the property of Eli Lilly and Company or its subsidiaries and should not be copied by or distributed to persons not involved in the clinical investigation of Necitumumab (IMC-11F8; LY3012211), unless such persons are bound by a confidentiality agreement with Eli Lilly and Company or its subsidiaries. Note to Regulatory Authorities: This document may contain protected personal data and/or commercially confidential information exempt from public disclosure. Eli Lilly and Company requests consultation regarding release/redaction prior to any public release. In the United States, this document is subject to Freedom of Information Act (FOIA) Exemption 4 and may not be reproduced or otherwise disseminated without the written approval of Eli Lilly and Company or its subsidiaries. Necitumumab (IMC-11F8; LY3012211) Gemcitabine (LY188011) This is a Phase 1b/2 study in the first-line treatment of patients with advanced (Stage IV) Squamous Non-Small Cell Lung Cancer (NSCLC). -

Advances in Epidermal Growth Factor Receptor Specific Immunotherapy: Lessons to Be Learned from Armed Antibodies
www.oncotarget.com Oncotarget, 2020, Vol. 11, (No. 38), pp: 3531-3557 Review Advances in epidermal growth factor receptor specific immunotherapy: lessons to be learned from armed antibodies Fleury Augustin Nsole Biteghe1,*, Neelakshi Mungra2,*, Nyangone Ekome Toung Chalomie4, Jean De La Croix Ndong5, Jean Engohang-Ndong6, Guillaume Vignaux7, Eden Padayachee8, Krupa Naran2,* and Stefan Barth2,3,* 1Department of Radiation Oncology and Biomedical Sciences, Cedars-Sinai Medical, Los Angeles, CA, USA 2Medical Biotechnology & Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa 3South African Research Chair in Cancer Biotechnology, Department of Integrative Biomedical Sciences, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa 4Sun Yat-Sen University, Zhongshan Medical School, Guangzhou, China 5Department of Orthopedic Surgery, New York University School of Medicine, New York, NY, USA 6Department of Biological Sciences, Kent State University at Tuscarawas, New Philadelphia, OH, USA 7Arctic Slope Regional Corporation Federal, Beltsville, MD, USA 8Department of Physiology, University of Kentucky, Lexington, KY, USA *These authors contributed equally to this work Correspondence to: Stefan Barth, email: [email protected] Keywords: epidermal growth factor receptor (EGFR); recombinant immunotoxins (ITs); targeted human cytolytic fusion proteins (hCFPs); recombinant antibody-drug conjugates (rADCs); recombinant antibody photoimmunoconjugates (rAPCs) Received: May 30, 2020 Accepted: August 11, 2020 Published: September 22, 2020 Copyright: © 2020 Biteghe et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Interim Report for the First Quarter of 2020
Genmab Announces Financial Results for the First Quarter of 2020 May 6, 2020; Copenhagen, Denmark; Interim Report for the First Quarter Ended March 31, 2020 Highlights DARZALEX® (daratumumab) net sales increased approximately 49% compared to the first quarter of 2019 to USD 937 million, resulting in royalty income of DKK 775 million DARZALEX approved in Europe in combination with bortezomib, thalidomide and dexamethasone for the treatment of adult patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplant U.S. FDA approved TEPEZZA™ (teprotumumab-trbw), developed and commercialized by Horizon Therapeutics, for thyroid eye disease U.S. FDA accepted, with priority review, Novartis’ supplemental Biologics License Application for subcutaneous ofatumumab in relapsing multiple sclerosis Anthony Pagano appointed Chief Financial Officer Anthony Mancini appointed Chief Operating Officer “Despite the unprecedented challenges posed by the coronavirus (COVID-19) pandemic, we will continue to invest in our innovative proprietary products, technologies and capabilities and use our world-class expertise in antibody drug development to create truly differentiated products with the potential to help cancer patients. While Genmab is closely monitoring the developments in the rapidly evolving landscape, we are extremely fortunate to have a solid financial foundation and a fabulous and committed team to carry us through these uncertain times,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. Financial Performance First Quarter of 2020 Revenue was DKK 892 million in the first quarter of 2020 compared to DKK 591 million in the first quarter of 2019. The increase of DKK 301 million, or 51%, was mainly driven by higher DARZALEX royalties. -

Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy
cancers Review Primary and Acquired Resistance to Immunotherapy in Lung Cancer: Unveiling the Mechanisms Underlying of Immune Checkpoint Blockade Therapy Laura Boyero 1 , Amparo Sánchez-Gastaldo 2, Miriam Alonso 2, 1 1,2,3, , 1,2, , José Francisco Noguera-Uclés , Sonia Molina-Pinelo * y and Reyes Bernabé-Caro * y 1 Institute of Biomedicine of Seville (IBiS) (HUVR, CSIC, Universidad de Sevilla), 41013 Seville, Spain; [email protected] (L.B.); [email protected] (J.F.N.-U.) 2 Medical Oncology Department, Hospital Universitario Virgen del Rocio, 41013 Seville, Spain; [email protected] (A.S.-G.); [email protected] (M.A.) 3 Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), 28029 Madrid, Spain * Correspondence: [email protected] (S.M.-P.); [email protected] (R.B.-C.) These authors contributed equally to this work. y Received: 16 November 2020; Accepted: 9 December 2020; Published: 11 December 2020 Simple Summary: Immuno-oncology has redefined the treatment of lung cancer, with the ultimate goal being the reactivation of the anti-tumor immune response. This has led to the development of several therapeutic strategies focused in this direction. However, a high percentage of lung cancer patients do not respond to these therapies or their responses are transient. Here, we summarized the impact of immunotherapy on lung cancer patients in the latest clinical trials conducted on this disease. As well as the mechanisms of primary and acquired resistance to immunotherapy in this disease. Abstract: After several decades without maintained responses or long-term survival of patients with lung cancer, novel therapies have emerged as a hopeful milestone in this research field. -
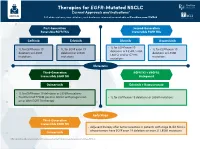
Therapies for EGFR-Mutated NSCLC Current Approvals and Indications1 Full Abbreviations, Accreditation, and Disclosure Information Available at Peerview.Com/CWE40
Therapies for EGFR-Mutated NSCLC 1 Current Approvals and Indications Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 First-Generation Second-Generation Reversible EGFR TKIs Irreversible EGFR TKIs Getinib Erlotinib Afatinib Dacomitinib • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 deletions or L858R, S768I, deletions or L858R deletions or L858R deletions or L858R L861Q, and/or G719X mutations mutations mutations mutations Metastatic Third-Generation EGFR TKI + VEGFR2 Irreversible EGFR TKI Antagonist Osimertinib Erlotinib + Ramucirumab • 1L for EGFR exon 19 deletions or L858R mutations • Treatment of T790M-positive NSCLC with progression • 1L for EGFR exon 19 deletions or L858R mutations on or after EGFR TKI therapy Early Stage Third-Generation Irreversible EGFR TKI • Adjuvant therapy after tumor resection in patients with stage IB-IIIA NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations Osimertinib 1. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Molecular Testing Guidelines for NSCLC Latest Updates, Best Practices, and Patient-Reported Insights1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 Why Test Lung Cancer Patients for Genomic Alterations? • Genomic alterations are common in nonsquamous NSCLC (approximately 50%) • Targeted therapies produce better treatment outcomes (eg, higher response rates, improved -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency. -

Necitumumab: a New Therapeutic Option for Squamous Cell Lung Cancer?
Editorial Necitumumab: a new therapeutic option for squamous cell lung cancer? Rathi N. Pillai, Suresh S. Ramalingam Winship Cancer Institute, Emory University, Atlanta, GA, USA Correspondence to: Suresh S. Ramalingam. Professor, Director of Medical Oncology, 1365 Clifton Road NE, Rm C-3090, Atlanta, GA 30322, USA. Email: [email protected]. Submitted Oct 03, 2014. Accepted for publication Oct 08, 2014. doi: 10.3978/j.issn.2218-6751.2014.11.01 View this article at: http://dx.doi.org/10.3978/j.issn.2218-6751.2014.11.01 Squamous cell carcinoma (SqCC) accounts for 25% Society of Clinical Oncology (ASCO) this year. This is the to 30% of all non-small lung carcinoma (NSCLC) largest study to date for patients with SqCC of the lung (3). diagnosed worldwide. Therapeutic advances for squamous A total of 1,093 patients with metastatic SqCC of the lung cell lung carcinoma have remained stagnant. This is in were randomized to treatment with gemcitabine/cisplatin stark comparison to adenocarcinoma of the lung, which combination given with or without necitumumab 800 mg has benefited from the development of therapies such intravenously on days 1 and 8 of a 21-day cycle. Patients as bevacizumab and pemetrexed, as well as targeted could receive up to six cycles of combination therapy therapies for specific molecular subsets with epidermal and then continue on maintenance necitumumab if they growth factor receptor (EGFR) mutations and anaplastic achieved clinical benefit. The primary endpoint was overall lymphoma kinase (ALK) rearrangements. Recent genomic survival (OS), with a pre-planned exploratory endpoint of characterization of SqCC by The Cancer Genome Atlas the use of EGFR protein expression as measured by H-score has identified mutations/amplifications in receptor tyrosine as a predictive biomarker. -

Anti-EGFR Antibody 528 Binds to Domain III of EGFR at a Site
www.nature.com/scientificreports OPEN Anti‑EGFR antibody 528 binds to domain III of EGFR at a site shifted from the cetuximab epitope Koki Makabe1, Takeshi Yokoyama2,3, Shiro Uehara2, Tomomi Uchikubo‑Kamo3, Mikako Shirouzu3, Kouki Kimura4, Kouhei Tsumoto5,6, Ryutaro Asano4, Yoshikazu Tanaka2 & Izumi Kumagai4* Antibodies have been widely used for cancer therapy owing to their ability to distinguish cancer cells by recognizing cancer‑specifc antigens. Epidermal growth factor receptor (EGFR) is a promising target for the cancer therapeutics, against which several antibody clones have been developed and brought into therapeutic use. Another antibody clone, 528, is an antagonistic anti‑EGFR antibody, which has been the focus of our antibody engineering studies to develop cancer drugs. In this study, we explored the interaction of 528 with the extracellular region of EGFR (sEGFR) via binding analyses and structural studies. Dot blotting experiments with heat treated sEGFR and surface plasmon resonance binding experiments revealed that 528 recognizes the tertiary structure of sEGFR and exhibits competitive binding to sEGFR with EGF and cetuximab. Single particle analysis of the sEGFR–528 Fab complex via electron microscopy clearly showed the binding of 528 to domain III of sEGFR, the domain to which EGF and cetuximab bind, explaining its antagonistic activity. Comparison between the two‑ dimensional class average and the cetuximab/sEGFR crystal structure revealed that 528 binds to a site that is shifted from, rather than identical to, the cetuximab epitope, and may exclude known drug‑ resistant EGFR mutations. Epidermal growth factor receptor (EGFR) is a member of the closely related family of ErbB transmembrane protein tyrosine kinase receptors. -

CDER Therapeutic Biologic Products List
CDER Therapeutic Biologic Products This list is intended to include all the Center for Drug Evaluation and Research (CDER) user fee billable therapeutic biological products and potencies approved under Section 351 of the Public Health Service Act. The Orange Book includes a section entitled "Drug Products with Approval under Section 505 of the Act Administered by CBER." Included on that list are several products that have been transferred to CDER which would be considered billable also. Program fees are assessed for each potency in which the approved (non-revoked, non-suspended) product is manufactured in final dosage form. When evaluating the specific strength or potency of a drug in final dosage form for purposes of assessing program fees for liquid parenteral biological products, CDER intends to take into consideration both the total amount of drug substance in mass or units of activity in a product and the concentration of drug substance (mass or units of activity per unit volume of product). Biologic products considered to have a different strength or potency in a final dosage form will be given separate entries in the Biologics List and assessed separate program fees. An auto-injector that has the same strength or potency as a prefilled syringe or vial will generally be assessed a separate prescription drug program fee. In certain circumstances, products which have been discontinued from marketing but are still licensed are not assessed program fees. Those products are identified on the CDER Discontinued Biologic Product List section. The potency information contained in this list is based on information in our database. -

In This Issue
IN THIS ISSUE PD-1 Blockade Is Not Unsafe in Patients with Lung Cancer and COVID-19 • COVID-19 severity and mortality were not • Although PD-1 blockade was not a • This suggests that PD-1 blockade increased in patients with lung cancer risk factor in this population, severity should be used when indicated in patients who received prior PD-1 blockade . and mortality in this group were high . with lung cancer despite COVID-19 . A key issue in oncology of whether patients received the immunotherapy recently or practice during the COVID-19 at any point prior to infection. There was a slight, statistically pandemic is whether PD-1 insignifi cant numeric increase in severity with PD-1 blockade, blockade affects the severity of but controlling for smoking history—which is an expected COVID-19 in patients with can- imbalance in receipt of prior PD-1 blockade and a risk factor cer. PD-1 blockade may increase for severe COVID-19 outcomes—abolished this correlation. COVID-19 severity by contrib- This work suggests that prior PD-1 blockade is not a clinically uting to hyperactive immune meaningful risk factor for worsened COVID-19 outcomes, res ponses to SARS-CoV-2 infec- implying that PD-1 blockade should be used when indicated tion—or, alternatively, may reduce despite the pandemic. Larger studies are needed to more fully severity by enhancing control of initial viral infection. To examine this question. Finally, it should be noted that, in minimize lung cancer as a confounder, Luo and colleagues this study population, more than half of patients with lung assessed the outcomes of concurrent COVID-19 and lung cancer and COVID-19 were hospitalized and almost half of cancer in 69 consecutive patients at a single institution in those hospitalized died, emphasizing the need for studies to New York City. -

Antibodies for the Treatment of Brain Metastases, a Dream Or a Reality?
pharmaceutics Review Antibodies for the Treatment of Brain Metastases, a Dream or a Reality? Marco Cavaco, Diana Gaspar, Miguel ARB Castanho * and Vera Neves * Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-028 Lisboa, Portugal * Correspondence: [email protected] (M.A.R.B.C.); [email protected] (V.N.) Received: 19 November 2019; Accepted: 28 December 2019; Published: 13 January 2020 Abstract: The incidence of brain metastases (BM) in cancer patients is increasing. After diagnosis, overall survival (OS) is poor, elicited by the lack of an effective treatment. Monoclonal antibody (mAb)-based therapy has achieved remarkable success in treating both hematologic and non-central-nervous system (CNS) tumors due to their inherent targeting specificity. However, the use of mAbs in the treatment of CNS tumors is restricted by the blood–brain barrier (BBB) that hinders the delivery of either small-molecules drugs (sMDs) or therapeutic proteins (TPs). To overcome this limitation, active research is focused on the development of strategies to deliver TPs and increase their concentration in the brain. Yet, their molecular weight and hydrophilic nature turn this task into a challenge. The use of BBB peptide shuttles is an elegant strategy. They explore either receptor-mediated transcytosis (RMT) or adsorptive-mediated transcytosis (AMT) to cross the BBB. The latter is preferable since it avoids enzymatic degradation, receptor saturation, and competition with natural receptor substrates, which reduces adverse events. Therefore, the combination of mAbs properties (e.g., selectivity and long half-life) with BBB peptide shuttles (e.g., BBB translocation and delivery into the brain) turns the therapeutic conjugate in a valid approach to safely overcome the BBB and efficiently eliminate metastatic brain cells. -
![Full Prescribing Information [FDA]](https://docslib.b-cdn.net/cover/8240/full-prescribing-information-fda-1598240.webp)
Full Prescribing Information [FDA]
HIGHLIGHTS OF PRESCRIBING INFORMATION -----------------------------CONTRAINDICATIONS--------------------------------- These highlights do not include all the information needed to use None. (4) RYBREVANT™ safely and effectively. See full prescribing information -------------------------WARNINGS AND PRECAUTIONS---------------------- for RYBREVANT. Infusion-Related Reactions (IRR): Interrupt infusion at the first sign of RYBREVANT (amivantamab-vmjw) injection, for intravenous use IRRs. Reduce infusion rate or permanently discontinue RYBREVANT Initial U.S. Approval: 2021 based on severity. (2.4, 5.1) Interstitial Lung Disease (ILD)/Pneumonitis: Monitor for new or worsening symptoms indicative of ILD. Immediately withhold RYBREVANT in -----------------------------INDICATIONS AND USAGE--------------------------- patients with suspected ILD/pneumonitis and permanently discontinue if RYBREVANT is a bispecific EGF receptor-directed and MET receptor- ILD/pneumonitis is confirmed. (2.4, 5.2) directed antibody indicated for the treatment of adult patients with locally Dermatologic Adverse Reactions: May cause rash including acneiform advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal dermatitis and toxic epidermal necrolysis. Withhold, dose reduce or growth factor receptor (EGFR) exon 20 insertion mutations, as detected by an permanently discontinue RYBREVANT based on severity. (2.4, 5.3) FDA-approved test, whose disease has progressed on or after platinum-based Ocular Toxicity: Promptly refer patients with worsening eye symptoms to chemotherapy. (1, 2.1) an ophthalmologist. Withhold, dose reduce or permanently discontinue This indication is approved under accelerated approval based on overall RYBREVANT based on severity. (5.4) response rate and duration of response. Continued approval for this indication Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of may be contingent upon verification and description of clinical benefit in the reproductive potential of the potential risk to the fetus and to use effective confirmatory trials.