Brief Report Preclinical Characterization of Mobocertinib
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Press Release
Press Release Daiichi Sankyo and AstraZeneca Announce Global Development and Commercialization Collaboration for Daiichi Sankyo’s HER2 Targeting Antibody Drug Conjugate [Fam-] Trastuzumab Deruxtecan (DS-8201) Collaboration combines Daiichi Sankyo’s scientific and technological excellence with AstraZeneca’s global experience and resources in oncology to accelerate and expand the potential of [fam-] trastuzumab deruxtecan as monotherapy and combination therapy across a spectrum of HER2 expressing cancers AstraZeneca to pay Daiichi Sankyo up to $6.90 billion in total consideration, including $1.35 billion upfront payment and up to an additional $5.55 billion contingent upon achievement of future regulatory and sales milestones as well as other contingencies Companies to share equally development and commercialization costs as well as profits worldwide from [fam-] trastuzumab deruxtecan with Daiichi Sankyo maintaining exclusive rights in Japan Daiichi Sankyo is expected to book sales in U.S., certain countries in Europe, and certain other markets where Daiichi Sankyo has affiliates; AstraZeneca is expected to book sales in all other markets worldwide, including China, Australia, Canada and Russia Tokyo, Munich and Basking Ridge, NJ – (March 28, 2019) – Daiichi Sankyo Company, Limited (hereafter, Daiichi Sankyo) announced today that it has entered into a global development and commercialization agreement with AstraZeneca for Daiichi Sankyo’s lead antibody drug conjugate (ADC), [fam-] trastuzumab deruxtecan (DS-8201), currently in pivotal development for multiple HER2 expressing cancers including breast and gastric cancer, and additional development in non-small cell lung and colorectal cancer. Daiichi Sankyo and AstraZeneca will jointly develop and commercialize [fam-] trastuzumab deruxtecan as a monotherapy or a combination therapy worldwide, except in Japan where Daiichi Sankyo will maintain exclusive rights. -

Fam-Trastuzumab Deruxtecan-Nxki Submitted by Daiichi
Dan Liang, PharmD Associate Director, Medical Information & Education Daiichi Sankyo, Inc. 211 Mount Airy Road Basking Ridge, NJ 07920 Phone: 908-992-7054 Email: [email protected] Date of request: July 9, 2020 NCCN Panel: Colon Cancer and Rectal Cancer On behalf of Daiichi Sankyo, Inc. and AstraZeneca Pharmaceuticals LP, I respectfully request the NCCN Guideline Panel for Colon and Rectal Cancers to review data from the clinical study1 in support of fam-trastuzumab deruxtecan-nxki, also known as T-DXd, as a monotherapy option for the treatment of patients with HER2-positive unresectable and/or metastatic colorectal cancer. Specific Changes: We respectfully ask the NCCN panel to consider the following: • COL-D1 through COL-D6 and REC-F1 through REC-F6, “Continuum of Care - Systemic Therapy for Advanced or Metastatic Disease” o Add “Fam-trastuzumab deruxtecan-nxki (HER2-positive and RAS and BRAF WT)” to the following: . COL-D1 and REC-F1: “Patient not appropriate for intensive therapy” . COL-D2 and REC-F2: “Previous oxaliplatin-based therapy without irinotecan” . COL-D3 and REC-F3: “Previous irinotecan-based therapy without oxaliplatin” . COL-D4 and REC-F4: “Previous treatment with oxaliplatin and irinotecan” . COL-D5 and REC-F5: “Previous therapy without irinotecan or oxaliplatin” . COL-D6 and REC-F6: “FOLFOX or CAPEOX or (FOLFOX or CAPEOX) + bevacizumab” • COL-D11 and REC-F11, “Systemic Therapy for Advanced or Metastatic Disease – Chemotherapy Regimens” o Add “Fam-trastuzumab deruxtecan-nxki 6.4 mg/kg IV on Day 1, cycled every 21 days” with a footnote: “fam-trastuzumab deruxtecan-nxki is approved for metastatic HER2-positive breast cancer at a different dose of 5.4 mg/kg IV on Day 1, cycled every 21 days” FDA Clearance: ENHERTU (fam-trastuzumab deruxtecan-nxki) is a HER2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with unresectable or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting. -

Antibody–Drug Conjugates
Published OnlineFirst April 12, 2019; DOI: 10.1158/1078-0432.CCR-19-0272 Review Clinical Cancer Research Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index Steven Coats1, Marna Williams1, Benjamin Kebble1, Rakesh Dixit1, Leo Tseng1, Nai-Shun Yao1, David A. Tice1, and Jean-Charles Soria1,2 Abstract Since the first approval of gemtuzumab ozogamicin nism of activity of the cytotoxic warhead. However, the (Mylotarg; Pfizer; CD33 targeted), two additional antibody– enthusiasm to develop ADCs has not been dampened; drug conjugates (ADC), brentuximab vedotin (Adcetris; Seat- approximately 80 ADCs are in clinical development in tle Genetics, Inc.; CD30 targeted) and inotuzumab ozogami- nearly 600 clinical trials, and 2 to 3 novel ADCs are likely cin (Besponsa; Pfizer; CD22 targeted), have been approved for to be approved within the next few years. While the hematologic cancers and 1 ADC, trastuzumab emtansine promise of a more targeted chemotherapy with less tox- (Kadcyla; Genentech; HER2 targeted), has been approved to icity has not yet been realized with ADCs, improvements treat breast cancer. Despite a clear clinical benefit being dem- in technology combined with a wealth of clinical data are onstrated for all 4 approved ADCs, the toxicity profiles are helping to shape the future development of ADCs. In this comparable with those of standard-of-care chemotherapeu- review, we discuss the clinical and translational strategies tics, with dose-limiting toxicities associated with the mecha- associated with improving the therapeutic index for ADCs. Introduction in antibody, linker, and warhead technologies in significant depth (2, 3, 8, 9). Antibody–drug conjugates (ADC) were initially designed to leverage the exquisite specificity of antibodies to deliver targeted potent chemotherapeutic agents with the intention of improving Overview of ADCs in Clinical Development the therapeutic index (the ratio between the toxic dose and the Four ADCs have been approved over the last 20 years (Fig. -

Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies
cancers Review Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies Karam Khaddour 1,*, Sushma Jonna 1, Alexander Deneka 2 , Jyoti D. Patel 3, Mohamed E. Abazeed 4, Erica Golemis 2 , Hossein Borghaei 5 and Yanis Boumber 3,6,* 1 Division of Hematology and Oncology, University of Illinois at Chicago, Chicago, IL 60612, USA; [email protected] 2 Fox Chase Cancer Center, Program in Molecular Therapeutics, Philadelphia, PA 19111, USA; [email protected] (A.D.); [email protected] (E.G.) 3 Robert H. Lurie Comprehensive Cancer Center, Division of Hematology/Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 4 Robert H. Lurie Comprehensive Cancer Center, Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 5 Fox Chase Cancer Center, Department of Hematology and Oncology, Philadelphia, PA 19111, USA; [email protected] 6 Institute of Fundamental Medicine and Biology, Kazan Federal University, 420008 Kazan, Russia * Correspondence: [email protected] (K.K.); [email protected] (Y.B.) Simple Summary: Epidermal growth factor receptor (EGFR) mutations occur in a significant number Citation: Khaddour, K.; Jonna, S.; of lung cancer patients. Treatment outcomes in this subset of patients has greatly improved over the Deneka, A.; Patel, J.D.; Abazeed, M.E.; last decade after the introduction of EGFR tyrosine kinase inhibitors (TKIs), which demonstrated high Golemis, E.; Borghaei, H.; Boumber, Y. efficacy and improved survival in randomized clinical trials. Although EGFR TKIs became the stan- Targeting the Epidermal Growth dard of care in patients with EGFR-mutated lung cancer, resistance almost inevitably develops. -

Advances in Epidermal Growth Factor Receptor Specific Immunotherapy: Lessons to Be Learned from Armed Antibodies
www.oncotarget.com Oncotarget, 2020, Vol. 11, (No. 38), pp: 3531-3557 Review Advances in epidermal growth factor receptor specific immunotherapy: lessons to be learned from armed antibodies Fleury Augustin Nsole Biteghe1,*, Neelakshi Mungra2,*, Nyangone Ekome Toung Chalomie4, Jean De La Croix Ndong5, Jean Engohang-Ndong6, Guillaume Vignaux7, Eden Padayachee8, Krupa Naran2,* and Stefan Barth2,3,* 1Department of Radiation Oncology and Biomedical Sciences, Cedars-Sinai Medical, Los Angeles, CA, USA 2Medical Biotechnology & Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa 3South African Research Chair in Cancer Biotechnology, Department of Integrative Biomedical Sciences, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa 4Sun Yat-Sen University, Zhongshan Medical School, Guangzhou, China 5Department of Orthopedic Surgery, New York University School of Medicine, New York, NY, USA 6Department of Biological Sciences, Kent State University at Tuscarawas, New Philadelphia, OH, USA 7Arctic Slope Regional Corporation Federal, Beltsville, MD, USA 8Department of Physiology, University of Kentucky, Lexington, KY, USA *These authors contributed equally to this work Correspondence to: Stefan Barth, email: [email protected] Keywords: epidermal growth factor receptor (EGFR); recombinant immunotoxins (ITs); targeted human cytolytic fusion proteins (hCFPs); recombinant antibody-drug conjugates (rADCs); recombinant antibody photoimmunoconjugates (rAPCs) Received: May 30, 2020 Accepted: August 11, 2020 Published: September 22, 2020 Copyright: © 2020 Biteghe et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Interim Report for the First Quarter of 2020
Genmab Announces Financial Results for the First Quarter of 2020 May 6, 2020; Copenhagen, Denmark; Interim Report for the First Quarter Ended March 31, 2020 Highlights DARZALEX® (daratumumab) net sales increased approximately 49% compared to the first quarter of 2019 to USD 937 million, resulting in royalty income of DKK 775 million DARZALEX approved in Europe in combination with bortezomib, thalidomide and dexamethasone for the treatment of adult patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplant U.S. FDA approved TEPEZZA™ (teprotumumab-trbw), developed and commercialized by Horizon Therapeutics, for thyroid eye disease U.S. FDA accepted, with priority review, Novartis’ supplemental Biologics License Application for subcutaneous ofatumumab in relapsing multiple sclerosis Anthony Pagano appointed Chief Financial Officer Anthony Mancini appointed Chief Operating Officer “Despite the unprecedented challenges posed by the coronavirus (COVID-19) pandemic, we will continue to invest in our innovative proprietary products, technologies and capabilities and use our world-class expertise in antibody drug development to create truly differentiated products with the potential to help cancer patients. While Genmab is closely monitoring the developments in the rapidly evolving landscape, we are extremely fortunate to have a solid financial foundation and a fabulous and committed team to carry us through these uncertain times,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. Financial Performance First Quarter of 2020 Revenue was DKK 892 million in the first quarter of 2020 compared to DKK 591 million in the first quarter of 2019. The increase of DKK 301 million, or 51%, was mainly driven by higher DARZALEX royalties. -
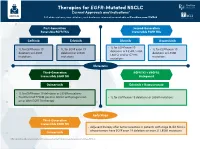
Therapies for EGFR-Mutated NSCLC Current Approvals and Indications1 Full Abbreviations, Accreditation, and Disclosure Information Available at Peerview.Com/CWE40
Therapies for EGFR-Mutated NSCLC 1 Current Approvals and Indications Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 First-Generation Second-Generation Reversible EGFR TKIs Irreversible EGFR TKIs Getinib Erlotinib Afatinib Dacomitinib • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 deletions or L858R, S768I, deletions or L858R deletions or L858R deletions or L858R L861Q, and/or G719X mutations mutations mutations mutations Metastatic Third-Generation EGFR TKI + VEGFR2 Irreversible EGFR TKI Antagonist Osimertinib Erlotinib + Ramucirumab • 1L for EGFR exon 19 deletions or L858R mutations • Treatment of T790M-positive NSCLC with progression • 1L for EGFR exon 19 deletions or L858R mutations on or after EGFR TKI therapy Early Stage Third-Generation Irreversible EGFR TKI • Adjuvant therapy after tumor resection in patients with stage IB-IIIA NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations Osimertinib 1. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Molecular Testing Guidelines for NSCLC Latest Updates, Best Practices, and Patient-Reported Insights1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 Why Test Lung Cancer Patients for Genomic Alterations? • Genomic alterations are common in nonsquamous NSCLC (approximately 50%) • Targeted therapies produce better treatment outcomes (eg, higher response rates, improved -

Antibody–Drug Conjugates: the Last Decade
pharmaceuticals Review Antibody–Drug Conjugates: The Last Decade Nicolas Joubert 1,* , Alain Beck 2 , Charles Dumontet 3,4 and Caroline Denevault-Sabourin 1 1 GICC EA7501, Equipe IMT, Université de Tours, UFR des Sciences Pharmaceutiques, 31 Avenue Monge, 37200 Tours, France; [email protected] 2 Institut de Recherche Pierre Fabre, Centre d’Immunologie Pierre Fabre, 5 Avenue Napoléon III, 74160 Saint Julien en Genevois, France; [email protected] 3 Cancer Research Center of Lyon (CRCL), INSERM, 1052/CNRS 5286/UCBL, 69000 Lyon, France; [email protected] 4 Hospices Civils de Lyon, 69000 Lyon, France * Correspondence: [email protected] Received: 17 August 2020; Accepted: 10 September 2020; Published: 14 September 2020 Abstract: An armed antibody (antibody–drug conjugate or ADC) is a vectorized chemotherapy, which results from the grafting of a cytotoxic agent onto a monoclonal antibody via a judiciously constructed spacer arm. ADCs have made considerable progress in 10 years. While in 2009 only gemtuzumab ozogamicin (Mylotarg®) was used clinically, in 2020, 9 Food and Drug Administration (FDA)-approved ADCs are available, and more than 80 others are in active clinical studies. This review will focus on FDA-approved and late-stage ADCs, their limitations including their toxicity and associated resistance mechanisms, as well as new emerging strategies to address these issues and attempt to widen their therapeutic window. Finally, we will discuss their combination with conventional chemotherapy or checkpoint inhibitors, and their design for applications beyond oncology, to make ADCs the magic bullet that Paul Ehrlich dreamed of. Keywords: antibody–drug conjugate; ADC; bioconjugation; linker; payload; cancer; resistance; combination therapies 1. -

Changing the Destiny of HER2 Expressing Solid Tumors
International Journal of Molecular Sciences Review Trastuzumab Deruxtecan: Changing the Destiny of HER2 Expressing Solid Tumors Alice Indini 1 , Erika Rijavec 1 and Francesco Grossi 2,* 1 Medical Oncology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (A.I.); [email protected] (E.R.) 2 Medical Oncology Unit, Department of Medicine and Surgery, University of Insubria, ASST dei Sette Laghi, 21100 Varese, Italy * Correspondence: [email protected] or [email protected] Abstract: HER2 targeted therapies have significantly improved prognosis of HER2-positive breast and gastric cancer. HER2 overexpression and mutation is the pathogenic driver in non-small cell lung cancer (NSCLC) and colorectal cancer, however, to date, there are no approved HER2-targeted therapies with these indications. Trastuzumab deruxtecan (T-DXd) is a novel HER2-directed antibody drug conjugate showing significant anti-tumor activity in heavily pre-treated HER2-positive breast and gastric cancer patients. Preliminary data have shown promising objective response rates in patients with HER2-positive NSCLC and colorectal cancer. T-DXd has an acceptable safety profile, however with concerns regarding potentially serious treatment-emergent adverse events. In this review we focus on the pharmacologic characteristics and toxicity profile of T-Dxd, and provide an update on the most recent results of clinical trials of T-DXd in solid tumors. The referenced papers were selected through a PubMed search performed on 16 March 2021 with the following searching terms: T-DXd and breast cancer, or gastric cancer, or non-small cell lung cancer (NSCLC), or colorectal cancer. -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency. -

In This Issue
IN THIS ISSUE PD-1 Blockade Is Not Unsafe in Patients with Lung Cancer and COVID-19 • COVID-19 severity and mortality were not • Although PD-1 blockade was not a • This suggests that PD-1 blockade increased in patients with lung cancer risk factor in this population, severity should be used when indicated in patients who received prior PD-1 blockade . and mortality in this group were high . with lung cancer despite COVID-19 . A key issue in oncology of whether patients received the immunotherapy recently or practice during the COVID-19 at any point prior to infection. There was a slight, statistically pandemic is whether PD-1 insignifi cant numeric increase in severity with PD-1 blockade, blockade affects the severity of but controlling for smoking history—which is an expected COVID-19 in patients with can- imbalance in receipt of prior PD-1 blockade and a risk factor cer. PD-1 blockade may increase for severe COVID-19 outcomes—abolished this correlation. COVID-19 severity by contrib- This work suggests that prior PD-1 blockade is not a clinically uting to hyperactive immune meaningful risk factor for worsened COVID-19 outcomes, res ponses to SARS-CoV-2 infec- implying that PD-1 blockade should be used when indicated tion—or, alternatively, may reduce despite the pandemic. Larger studies are needed to more fully severity by enhancing control of initial viral infection. To examine this question. Finally, it should be noted that, in minimize lung cancer as a confounder, Luo and colleagues this study population, more than half of patients with lung assessed the outcomes of concurrent COVID-19 and lung cancer and COVID-19 were hospitalized and almost half of cancer in 69 consecutive patients at a single institution in those hospitalized died, emphasizing the need for studies to New York City. -

Antibodies for the Treatment of Brain Metastases, a Dream Or a Reality?
pharmaceutics Review Antibodies for the Treatment of Brain Metastases, a Dream or a Reality? Marco Cavaco, Diana Gaspar, Miguel ARB Castanho * and Vera Neves * Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Av. Prof. Egas Moniz, 1649-028 Lisboa, Portugal * Correspondence: [email protected] (M.A.R.B.C.); [email protected] (V.N.) Received: 19 November 2019; Accepted: 28 December 2019; Published: 13 January 2020 Abstract: The incidence of brain metastases (BM) in cancer patients is increasing. After diagnosis, overall survival (OS) is poor, elicited by the lack of an effective treatment. Monoclonal antibody (mAb)-based therapy has achieved remarkable success in treating both hematologic and non-central-nervous system (CNS) tumors due to their inherent targeting specificity. However, the use of mAbs in the treatment of CNS tumors is restricted by the blood–brain barrier (BBB) that hinders the delivery of either small-molecules drugs (sMDs) or therapeutic proteins (TPs). To overcome this limitation, active research is focused on the development of strategies to deliver TPs and increase their concentration in the brain. Yet, their molecular weight and hydrophilic nature turn this task into a challenge. The use of BBB peptide shuttles is an elegant strategy. They explore either receptor-mediated transcytosis (RMT) or adsorptive-mediated transcytosis (AMT) to cross the BBB. The latter is preferable since it avoids enzymatic degradation, receptor saturation, and competition with natural receptor substrates, which reduces adverse events. Therefore, the combination of mAbs properties (e.g., selectivity and long half-life) with BBB peptide shuttles (e.g., BBB translocation and delivery into the brain) turns the therapeutic conjugate in a valid approach to safely overcome the BBB and efficiently eliminate metastatic brain cells.