Interim Report for the First Quarter of 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacokinetics and Exposure-Response Relationship of Teprotumumab Frst 3 Months, Unless Determined to Be Medically Necessary
Clinical Pharmacokinetics https://doi.org/10.1007/s40262-021-01003-3 ORIGINAL RESEARCH ARTICLE Pharmacokinetics and Exposure‑Response Relationship of Teprotumumab, an Insulin‑Like Growth Factor‑1 Receptor‑Blocking Antibody, in Thyroid Eye Disease Yan Xin1 · Fengyan Xu2 · Yuying Gao2 · Nivedita Bhatt1 · Jason Chamberlain1 · Saba Sile1 · Suzy Hammel1 · Robert J. Holt1 · Srini Ramanathan1 Accepted: 10 February 2021 © The Author(s) 2021 Abstract Background and Objective Thyroid eye disease (TED) is characterized by infammation/expansion of orbital tissues, prop- tosis, and diplopia. Teprotumumab is the frst US Food and Drug Administration-approved therapy for TED, administered as an initial intravenous infusion of 10 mg/kg followed by 20 mg/kg every 3 weeks for an additional seven infusions. The objec- tive of this article is to discuss the pharmacokinetics and exposure-response profle for teprotumumab in patients with TED. Methods A population pharmacokinetic analysis was performed to characterize pharmacokinetics and select dosing in patients with TED. Exposure-response was evaluated for efcacy (proptosis response, clinical activity score categorical response, and diplopia response) and safety (hyperglycemia, muscle spasms, and hearing impairment) parameters. Results Teprotumumab pharmacokinetics was linear in patients with TED, with low systemic clearance (0.334 L/day), low volume of distribution (3.9 and 4.2 L for the central and peripheral compartment, respectively), and a long elimination half- life (19.9 days). The approved dosing regimen provided > 20 µg/mL for > 90% insulin-like growth factor 1 receptor saturation throughout the dosing interval. Model-predicted mean (± standard deviation) steady-state area under the concentration-time curve, peak, and trough concentrations in patients with TED were 131 (± 30.9) mg∙h/mL, 643 (± 130) µg/mL, and 157 (± 50.6) µg/mL, respectively. -

Genmab Announces Data to Be Presented at 2017 ASCO Annual Meeting
Genmab Announces Data to be Presented at 2017 ASCO Annual Meeting Media Release 8 abstracts on Genmab programs scheduled for presentation at ASCO Two daratumumab oral presentations and five daratumumab poster presentations Copenhagen, Denmark; April 20, 2017 – Genmab A/S (Nasdaq Copenhagen: GEN) announced today that seven daratumumab abstracts have been accepted for presentation at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, June 2 – 6. These abstracts, submitted by our collaboration partner, Janssen Biotech, Inc., include updates for the POLLUX and CASTOR trials, and the first data for a Phase I study evaluating daratumumab with carfilzomib, lenalidomide and dexamethasone in front line multiple myeloma patients, which will be presented in an oral presentation. In addition, descriptions of the Phase Ib/II study of daratumumab plus atezolizumab in non-small cell lung cancer and of our Phase I/II study with HuMax-AXL-ADC are scheduled for poster presentations at the meeting. The titles of the abstracts are currently available on the ASCO website with the full abstracts scheduled to be published on May 17, 2017. “We are very pleased that, once again, a number of abstracts based on exciting work with Genmab’s innovative therapeutic antibody products have been accepted for presentation at the prestigious ASCO conference,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. List of abstracts: Daratumumab: Efficacy Of Daratumumab In Combination with Lenalidomide Plus Dexamethasone (DRd) or -

Genmab's 2020 Capital Markets
WELCOME Genmab’s 2020 Capital Markets Day November 13, 2020 Webcast Live from Utrecht and Princeton Forward Looking Statement This presentation contains forward looking statements. The words “believe”, “expect”, “anticipate”, “intend” and “plan” and similar expressions identify forward looking statements. All statements other than statements of historical facts included in this presentation, including, without limitation, those regarding our financial position, business strategy, plans and objectives of management for future operations (including development plans and objectives relating to our products), are forward looking statements. Such forward looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward looking statements. Such forward looking statements are based on numerous assumptions regarding our present and future business strategies and the environment in which we will operate in the future. The important factors that could cause our actual results, performance or achievements to differ materially from those in the forward looking statements include, among others, risks associated with product discovery and development, uncertainties related to the outcome of clinical trials, slower than expected rates of patient recruitment, unforeseen safety issues resulting from the administration of our products in patients, uncertainties related to product manufacturing, the lack of market acceptance of our products, our inability to manage growth, the competitive environment in relation to our business area and markets, our inability to attract and retain suitably qualified personnel, the unenforceability or lack of protection of our patents and proprietary rights, our relationships with affiliated entities, changes and developments in technology which may render our products obsolete, and other factors. -

Second Pre-Clinical Milestone Met in Lundbeck Collaboration - €1 Million Milestone Payment to Genmab
GENMAB REACHES SECOND MILESTONE IN LUNDBECK COLLABORATION - Second pre-clinical milestone met in Lundbeck collaboration - €1 million milestone payment to Genmab Copenhagen, Denmark; February 10, 2012 – Genmab A/S (OMX: GEN) announced today it had reached the second pre-clinical milestone in the collaboration with H. Lundbeck A/S, triggering a €1 million payment. Genmab has reached the second milestone in the collaboration with H. Lundbeck A/S to create and develop human antibody therapeutics for disorders of the central nervous system (CNS). The milestone triggers a payment of €1 million to Genmab. Under the collaboration with Lundbeck Genmab creates novel human antibodies to three targets identified by Lundbeck and Lundbeck has access to Genmab’s antibody creation and development capabilities, including its state of the art, fully automated pre-clinical antibody screening and characterization capabilities and its proprietary stabilized IgG4 and UniBody therapeutic antibody platforms. Under the terms of the agreement, Genmab received an upfront payment of €7.5 million in October 2010 (approximately DKK 56 million). Lundbeck fully funds the development of the antibodies. If all milestones in the agreement are achieved, the total value of the agreement to Genmab would be approximately €38 million (approximately DKK 283 million), plus single-digit royalties. “We are very pleased to have met the in vitro proof of concept milestone for another target in the Lundbeck collaboration. This partnership is progressing well, with this second milestone coming shortly after we achieved the first preclinical milestone in December last year,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. -

Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria
Oregon Medicaid Pharmaceutical Services Prior Authorization Criteria HEALTH SYSTEMS DIVISION Prior authorization (PA) criteria for fee-for-service prescriptions for Oregon Health Plan clients March 1, 2021 Contents Contents ................................................................................................................................................................ 2 Introduction........................................................................................................................................................... 7 About this guide ......................................................................................................................................... 7 How to use this guide ................................................................................................................................. 7 Administrative rules and supplemental information .................................................................................. 7 Update information............................................................................................................................................... 8 Effective March 1, 2021 ............................................................................................................................ 8 Substantive updates and new criteria ............................................................................................. 8 Clerical changes ............................................................................................................................ -

IGF System in Sarcomas: a Crucial Pathway with Many Unknowns to Exploit for Therapy
61 1 Journal of Molecular C Mancarella and Targeting IGF system in sarcoma 61:1 T45–T60 Endocrinology K Scotlandi THEMATIC REVIEW 40 YEARS OF IGF1 IGF system in sarcomas: a crucial pathway with many unknowns to exploit for therapy Caterina Mancarella and Katia Scotlandi Experimental Oncology Lab, CRS Development of Biomolecular Therapies, Orthopaedic Rizzoli Institute, Bologna, Italy Correspondence should be addressed to K Scotlandi: [email protected] This paper forms part of a special section on 40 Years of IGF1. The guest editors for this section were Derek LeRoith and Emily Gallagher. Abstract The insulin-like growth factor (IGF) system has gained substantial interest due to its Key Words involvement in regulating cell proliferation, differentiation and survival during anoikis f sarcomas and after conventional and targeted therapies. However, results from clinical trials have f IGF system been largely disappointing, with only a few but notable exceptions, such as trials targeting f targeted therapy sarcomas, especially Ewing sarcoma. This review highlights key studies focusing on IGF f clinical trials signaling in sarcomas, specifically studies underscoring the properties that make this system an attractive therapeutic target and identifies new relationships that may be exploited. This review discusses the potential roles of IGF2 mRNA-binding proteins (IGF2BPs), discoidin domain receptors (DDRs) and metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) in regulating the IGF system. Deeper investigation of these novel regulators of the IGF system may help us to further elucidate the spatial and temporal control of the IGF Journal of Molecular axis, as understanding the control of this axis is essential for future clinical studies. -

Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies
cancers Review Targeting the Epidermal Growth Factor Receptor in EGFR-Mutated Lung Cancer: Current and Emerging Therapies Karam Khaddour 1,*, Sushma Jonna 1, Alexander Deneka 2 , Jyoti D. Patel 3, Mohamed E. Abazeed 4, Erica Golemis 2 , Hossein Borghaei 5 and Yanis Boumber 3,6,* 1 Division of Hematology and Oncology, University of Illinois at Chicago, Chicago, IL 60612, USA; [email protected] 2 Fox Chase Cancer Center, Program in Molecular Therapeutics, Philadelphia, PA 19111, USA; [email protected] (A.D.); [email protected] (E.G.) 3 Robert H. Lurie Comprehensive Cancer Center, Division of Hematology/Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 4 Robert H. Lurie Comprehensive Cancer Center, Department of Radiation Oncology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611, USA; [email protected] 5 Fox Chase Cancer Center, Department of Hematology and Oncology, Philadelphia, PA 19111, USA; [email protected] 6 Institute of Fundamental Medicine and Biology, Kazan Federal University, 420008 Kazan, Russia * Correspondence: [email protected] (K.K.); [email protected] (Y.B.) Simple Summary: Epidermal growth factor receptor (EGFR) mutations occur in a significant number Citation: Khaddour, K.; Jonna, S.; of lung cancer patients. Treatment outcomes in this subset of patients has greatly improved over the Deneka, A.; Patel, J.D.; Abazeed, M.E.; last decade after the introduction of EGFR tyrosine kinase inhibitors (TKIs), which demonstrated high Golemis, E.; Borghaei, H.; Boumber, Y. efficacy and improved survival in randomized clinical trials. Although EGFR TKIs became the stan- Targeting the Epidermal Growth dard of care in patients with EGFR-mutated lung cancer, resistance almost inevitably develops. -

Therapeutic Targeting of the IGF Axis
cells Review Therapeutic Targeting of the IGF Axis Eliot Osher and Valentine M. Macaulay * Department of Oncology, University of Oxford, Oxford, OX3 7DQ, UK * Correspondence: [email protected]; Tel.: +44-1865617337 Received: 8 July 2019; Accepted: 9 August 2019; Published: 14 August 2019 Abstract: The insulin like growth factor (IGF) axis plays a fundamental role in normal growth and development, and when deregulated makes an important contribution to disease. Here, we review the functions mediated by ligand-induced IGF axis activation, and discuss the evidence for the involvement of IGF signaling in the pathogenesis of cancer, endocrine disorders including acromegaly, diabetes and thyroid eye disease, skin diseases such as acne and psoriasis, and the frailty that accompanies aging. We discuss the use of IGF axis inhibitors, focusing on the different approaches that have been taken to develop effective and tolerable ways to block this important signaling pathway. We outline the advantages and disadvantages of each approach, and discuss progress in evaluating these agents, including factors that contributed to the failure of many of these novel therapeutics in early phase cancer trials. Finally, we summarize grounds for cautious optimism for ongoing and future studies of IGF blockade in cancer and non-malignant disorders including thyroid eye disease and aging. Keywords: IGF; type 1 IGF receptor; IGF-1R; cancer; acromegaly; ophthalmopathy; IGF inhibitor 1. Introduction Insulin like growth factors (IGFs) are small (~7.5 kDa) ligands that play a critical role in many biological processes including proliferation and protection from apoptosis and normal somatic growth and development [1]. IGFs are members of a ligand family that includes insulin, a dipeptide comprised of A and B chains linked via two disulfide bonds, with a third disulfide linkage within the A chain. -
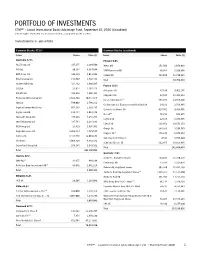
Portfolio of Investments
PORTFOLIO OF INVESTMENTS CTIVP® – Lazard International Equity Advantage Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.6% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 6.9% Finland 1.0% AGL Energy Ltd. 437,255 4,269,500 Metso OYJ 153,708 2,078,669 ASX Ltd. 80,181 4,687,834 UPM-Kymmene OYJ 36,364 1,106,808 BHP Group Ltd. 349,229 9,021,842 Valmet OYJ 469,080 11,570,861 Breville Group Ltd. 153,867 2,792,438 Total 14,756,338 Charter Hall Group 424,482 3,808,865 France 9.5% CSL Ltd. 21,611 4,464,114 Air Liquide SA 47,014 7,452,175 Data#3 Ltd. 392,648 1,866,463 Capgemini SE 88,945 11,411,232 Fortescue Metals Group Ltd. 2,622,808 30,812,817 Cie de Saint-Gobain(a) 595,105 24,927,266 IGO Ltd. 596,008 1,796,212 Cie Generale des Etablissements Michelin CSA 24,191 2,596,845 Ingenia Communities Group 665,283 2,191,435 Electricite de France SA 417,761 4,413,001 Kogan.com Ltd. 138,444 2,021,176 Elis SA(a) 76,713 968,415 Netwealth Group Ltd. 477,201 5,254,788 Legrand SA 22,398 1,783,985 Omni Bridgeway Ltd. 435,744 1,234,193 L’Oreal SA 119,452 38,873,153 REA Group Ltd. 23,810 1,895,961 Orange SA 298,281 3,106,763 Regis Resources Ltd. -
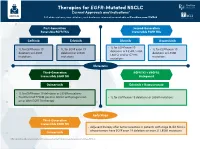
Therapies for EGFR-Mutated NSCLC Current Approvals and Indications1 Full Abbreviations, Accreditation, and Disclosure Information Available at Peerview.Com/CWE40
Therapies for EGFR-Mutated NSCLC 1 Current Approvals and Indications Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 First-Generation Second-Generation Reversible EGFR TKIs Irreversible EGFR TKIs Getinib Erlotinib Afatinib Dacomitinib • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 • 1L for EGFR exon 19 deletions or L858R, S768I, deletions or L858R deletions or L858R deletions or L858R L861Q, and/or G719X mutations mutations mutations mutations Metastatic Third-Generation EGFR TKI + VEGFR2 Irreversible EGFR TKI Antagonist Osimertinib Erlotinib + Ramucirumab • 1L for EGFR exon 19 deletions or L858R mutations • Treatment of T790M-positive NSCLC with progression • 1L for EGFR exon 19 deletions or L858R mutations on or after EGFR TKI therapy Early Stage Third-Generation Irreversible EGFR TKI • Adjuvant therapy after tumor resection in patients with stage IB-IIIA NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations Osimertinib 1. https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications. Molecular Testing Guidelines for NSCLC Latest Updates, Best Practices, and Patient-Reported Insights1 Full abbreviations, accreditation, and disclosure information available at PeerView.com/CWE40 Why Test Lung Cancer Patients for Genomic Alterations? • Genomic alterations are common in nonsquamous NSCLC (approximately 50%) • Targeted therapies produce better treatment outcomes (eg, higher response rates, improved -

Horizon Therapeutics Secures US FDA Approval For
Horizon Therapeutics Secures U.S. FDA Approval for Teprotumumab, for the Treatment of Thyroid Eye Disease (TED) Media Release Copenhagen, Denmark, January 23, 2020 • Third Genmab-created product approved by the U.S. FDA Genmab A/S (Nasdaq: GMAB) announced today that the U.S. Food and Drug Administration (U.S. FDA) has granted approval to Horizon Therapeutics (Nasdaq: HZNP) for the use of teprotumumab, under the trade name TEPEZZA™ (teprotumumab-trbw), for the treatment of Thyroid Eye Disease (TED). TEPEZZA, the first and only U.S. FDA-approved medicine for the treatment of TED, was developed by and is manufactured by Horizon, completing a long development program that began when Genmab created the molecule nearly two decades ago. Horizon submitted the Biologics License Application for teprotumumab, which received Priority Review, Orphan Drug, Fast Track and Breakthrough Therapy designations from the FDA. Teprotumumab was created by Genmab under a collaboration with Roche and development of the product is now being conducted by Horizon under a license from Roche. Under the terms of Genmab’s agreement with Roche, Genmab will receive mid- single digit royalties on sales of TEPEZZA. “We would like to congratulate Horizon on this exciting approval, and we are very pleased that patients in the United States living with thyroid eye disease, a vision-threatening autoimmune condition, will now have this important new treatment option,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. “We also believe that this approval, the third for a Genmab-created product following Arzerra® and DARZALEX®, reflects our company’s proven track record in improving the lives of patients by creating breakthrough innovative antibody products.” For more information about TEPEZZA and TED, please see the press release issued by Horizon Therapeutics. -

Portfolio of Investments
PORTFOLIO OF INVESTMENTS Variable Portfolio – Partners International Value Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.9% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 4.2% UCB SA 3,232 367,070 AMP Ltd. 247,119 232,705 Total 13,350,657 Aurizon Holdings Ltd. 64,744 199,177 China 0.6% Australia & New Zealand Banking Group Ltd. 340,950 4,253,691 Baidu, Inc., ADR(a) 15,000 1,898,850 Bendigo & Adelaide Bank Ltd. 30,812 134,198 China Mobile Ltd. 658,000 4,223,890 BlueScope Steel Ltd. 132,090 1,217,053 Total 6,122,740 Boral Ltd. 177,752 587,387 Denmark 1.9% Challenger Ltd. 802,400 2,232,907 AP Moller - Maersk A/S, Class A 160 234,206 Cleanaway Waste Management Ltd. 273,032 412,273 AP Moller - Maersk A/S, Class B 3,945 6,236,577 Crown Resorts Ltd. 31,489 200,032 Carlsberg A/S, Class B 12,199 1,643,476 Fortescue Metals Group Ltd. 194,057 2,279,787 Danske Bank A/S(a) 35,892 485,479 Harvey Norman Holdings Ltd. 144,797 471,278 Demant A/S(a) 8,210 257,475 Incitec Pivot Ltd. 377,247 552,746 Drilling Co. of 1972 A/S (The)(a) 40,700 879,052 LendLease Group 485,961 3,882,083 DSV PANALPINA A/S 15,851 2,571,083 Macquarie Group Ltd. 65,800 5,703,825 Genmab A/S(a) 1,071 388,672 National Australia Bank Ltd.