Genmab's 2020 Capital Markets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Genmab Announces Data to Be Presented at 2017 ASCO Annual Meeting
Genmab Announces Data to be Presented at 2017 ASCO Annual Meeting Media Release 8 abstracts on Genmab programs scheduled for presentation at ASCO Two daratumumab oral presentations and five daratumumab poster presentations Copenhagen, Denmark; April 20, 2017 – Genmab A/S (Nasdaq Copenhagen: GEN) announced today that seven daratumumab abstracts have been accepted for presentation at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, June 2 – 6. These abstracts, submitted by our collaboration partner, Janssen Biotech, Inc., include updates for the POLLUX and CASTOR trials, and the first data for a Phase I study evaluating daratumumab with carfilzomib, lenalidomide and dexamethasone in front line multiple myeloma patients, which will be presented in an oral presentation. In addition, descriptions of the Phase Ib/II study of daratumumab plus atezolizumab in non-small cell lung cancer and of our Phase I/II study with HuMax-AXL-ADC are scheduled for poster presentations at the meeting. The titles of the abstracts are currently available on the ASCO website with the full abstracts scheduled to be published on May 17, 2017. “We are very pleased that, once again, a number of abstracts based on exciting work with Genmab’s innovative therapeutic antibody products have been accepted for presentation at the prestigious ASCO conference,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. List of abstracts: Daratumumab: Efficacy Of Daratumumab In Combination with Lenalidomide Plus Dexamethasone (DRd) or -

Second Pre-Clinical Milestone Met in Lundbeck Collaboration - €1 Million Milestone Payment to Genmab
GENMAB REACHES SECOND MILESTONE IN LUNDBECK COLLABORATION - Second pre-clinical milestone met in Lundbeck collaboration - €1 million milestone payment to Genmab Copenhagen, Denmark; February 10, 2012 – Genmab A/S (OMX: GEN) announced today it had reached the second pre-clinical milestone in the collaboration with H. Lundbeck A/S, triggering a €1 million payment. Genmab has reached the second milestone in the collaboration with H. Lundbeck A/S to create and develop human antibody therapeutics for disorders of the central nervous system (CNS). The milestone triggers a payment of €1 million to Genmab. Under the collaboration with Lundbeck Genmab creates novel human antibodies to three targets identified by Lundbeck and Lundbeck has access to Genmab’s antibody creation and development capabilities, including its state of the art, fully automated pre-clinical antibody screening and characterization capabilities and its proprietary stabilized IgG4 and UniBody therapeutic antibody platforms. Under the terms of the agreement, Genmab received an upfront payment of €7.5 million in October 2010 (approximately DKK 56 million). Lundbeck fully funds the development of the antibodies. If all milestones in the agreement are achieved, the total value of the agreement to Genmab would be approximately €38 million (approximately DKK 283 million), plus single-digit royalties. “We are very pleased to have met the in vitro proof of concept milestone for another target in the Lundbeck collaboration. This partnership is progressing well, with this second milestone coming shortly after we achieved the first preclinical milestone in December last year,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. -

Interim Report for the First Quarter of 2020
Genmab Announces Financial Results for the First Quarter of 2020 May 6, 2020; Copenhagen, Denmark; Interim Report for the First Quarter Ended March 31, 2020 Highlights DARZALEX® (daratumumab) net sales increased approximately 49% compared to the first quarter of 2019 to USD 937 million, resulting in royalty income of DKK 775 million DARZALEX approved in Europe in combination with bortezomib, thalidomide and dexamethasone for the treatment of adult patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplant U.S. FDA approved TEPEZZA™ (teprotumumab-trbw), developed and commercialized by Horizon Therapeutics, for thyroid eye disease U.S. FDA accepted, with priority review, Novartis’ supplemental Biologics License Application for subcutaneous ofatumumab in relapsing multiple sclerosis Anthony Pagano appointed Chief Financial Officer Anthony Mancini appointed Chief Operating Officer “Despite the unprecedented challenges posed by the coronavirus (COVID-19) pandemic, we will continue to invest in our innovative proprietary products, technologies and capabilities and use our world-class expertise in antibody drug development to create truly differentiated products with the potential to help cancer patients. While Genmab is closely monitoring the developments in the rapidly evolving landscape, we are extremely fortunate to have a solid financial foundation and a fabulous and committed team to carry us through these uncertain times,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. Financial Performance First Quarter of 2020 Revenue was DKK 892 million in the first quarter of 2020 compared to DKK 591 million in the first quarter of 2019. The increase of DKK 301 million, or 51%, was mainly driven by higher DARZALEX royalties. -
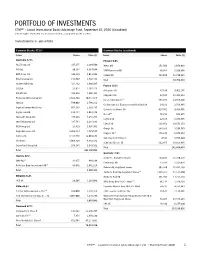
Portfolio of Investments
PORTFOLIO OF INVESTMENTS CTIVP® – Lazard International Equity Advantage Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.6% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 6.9% Finland 1.0% AGL Energy Ltd. 437,255 4,269,500 Metso OYJ 153,708 2,078,669 ASX Ltd. 80,181 4,687,834 UPM-Kymmene OYJ 36,364 1,106,808 BHP Group Ltd. 349,229 9,021,842 Valmet OYJ 469,080 11,570,861 Breville Group Ltd. 153,867 2,792,438 Total 14,756,338 Charter Hall Group 424,482 3,808,865 France 9.5% CSL Ltd. 21,611 4,464,114 Air Liquide SA 47,014 7,452,175 Data#3 Ltd. 392,648 1,866,463 Capgemini SE 88,945 11,411,232 Fortescue Metals Group Ltd. 2,622,808 30,812,817 Cie de Saint-Gobain(a) 595,105 24,927,266 IGO Ltd. 596,008 1,796,212 Cie Generale des Etablissements Michelin CSA 24,191 2,596,845 Ingenia Communities Group 665,283 2,191,435 Electricite de France SA 417,761 4,413,001 Kogan.com Ltd. 138,444 2,021,176 Elis SA(a) 76,713 968,415 Netwealth Group Ltd. 477,201 5,254,788 Legrand SA 22,398 1,783,985 Omni Bridgeway Ltd. 435,744 1,234,193 L’Oreal SA 119,452 38,873,153 REA Group Ltd. 23,810 1,895,961 Orange SA 298,281 3,106,763 Regis Resources Ltd. -

Portfolio of Investments
PORTFOLIO OF INVESTMENTS Variable Portfolio – Partners International Value Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.9% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 4.2% UCB SA 3,232 367,070 AMP Ltd. 247,119 232,705 Total 13,350,657 Aurizon Holdings Ltd. 64,744 199,177 China 0.6% Australia & New Zealand Banking Group Ltd. 340,950 4,253,691 Baidu, Inc., ADR(a) 15,000 1,898,850 Bendigo & Adelaide Bank Ltd. 30,812 134,198 China Mobile Ltd. 658,000 4,223,890 BlueScope Steel Ltd. 132,090 1,217,053 Total 6,122,740 Boral Ltd. 177,752 587,387 Denmark 1.9% Challenger Ltd. 802,400 2,232,907 AP Moller - Maersk A/S, Class A 160 234,206 Cleanaway Waste Management Ltd. 273,032 412,273 AP Moller - Maersk A/S, Class B 3,945 6,236,577 Crown Resorts Ltd. 31,489 200,032 Carlsberg A/S, Class B 12,199 1,643,476 Fortescue Metals Group Ltd. 194,057 2,279,787 Danske Bank A/S(a) 35,892 485,479 Harvey Norman Holdings Ltd. 144,797 471,278 Demant A/S(a) 8,210 257,475 Incitec Pivot Ltd. 377,247 552,746 Drilling Co. of 1972 A/S (The)(a) 40,700 879,052 LendLease Group 485,961 3,882,083 DSV PANALPINA A/S 15,851 2,571,083 Macquarie Group Ltd. 65,800 5,703,825 Genmab A/S(a) 1,071 388,672 National Australia Bank Ltd. -

Quarterly Commentary—Artisan Non-U.S. Small-Mid Growth Strategy
QUARTERLY Artisan Non-U.S. Small-Mid Growth Strategy FactCommentary Sheet As of 30 June 2019 Investment Process We seek long-term investments in high-quality businesses exposed to structural growth themes that can be acquired at sensible valuations in a contrarian fashion and are led by excellent management teams. Investing with Tailwinds We identify structural themes at the intersection of growth and change with the objective of investing in companies having meaningful exposure to these trends. Themes can be identified from both bottom-up and top-down perspectives. High-Quality Businesses We seek future leaders with attractive growth characteristics that we can own for the long term. Our fundamental analysis focuses on those companies exhibiting unique and defensible business models, high barriers to entry, proven management teams, favorable positions within their industry value chains and high or improving returns on capital. In short, we look to invest in small companies that have potential to become large. A Contrarian Approach to Valuation We seek to invest in high-quality businesses in a contrarian fashion. Mismatches between stock price and long-term business value are created by market dislocations, temporary slowdowns in individual businesses or misperceptions in the investment community. We also examine business transformation brought about by management change or restructuring. Manage Unique Risks of International Small- and Mid-Cap Equities International small- and mid-cap equities are exposed to unique investment risks that require managing. We define risk as permanent loss of capital, not share price volatility. We manage this risk by having a long-term ownership focus, understanding the direct and indirect security risks for each business, constructing the portfolio on a well-diversified basis and sizing positions according to individual risk characteristics. -
Citi Pure Growth Europe Long-Short Net TR Series II Index (CIISGRE2)
Date: 23-May-21 Index Weights as of monthly rebalance date 12-May-21 Citi Pure Growth Europe Long-Short Net TR Series II Index (CIISGRE2) Long Exposure Short Exposure Constituent Bloomberg Ticker Constituent Name Weight(%) Constituent Bloomberg Ticker Constituent Name Weight(%) 1 AAL LN Equity Anglo American Plc 1.62% 1 1COV GY Equity Covestro AG -0.89% 2 ACA FP Equity Credit Agricole SA 1.37% 2 ABBN SE Equity ABB LTD-REG -0.31% 3 ADM LN Equity Admiral Group 0.09% 3 ABF LN Equity Associated British Foods -0.62% 4 ADS GY Equity Adidas AG 1.01% 4 ABN NA Equity ABN AMRO Group NV -1.24% 5 ADYEN NA Equity Adyen NV 2.43% 5 AC FP Equity Accor -1.54% 6 AD NA Equity Ahold NV 0.61% 6 ADEN SE Equity ADECCO GROUP AG-REG -0.49% 7 AHT LN Equity Ashtead Group 0.24% 7 AENA SQ Equity AENA SA -1.05% 8 ALC SE Equity ALCON AG CHF0.04 0.70% 8 AGN NA Equity Aegon NV -0.71% 9 ALFA SS Equity Alfa Laval AB 0.11% 9 AI FP Equity Air Liquide -0.69% 10 ALO FP Equity Alstom 0.45% 10 AKZA NA Equity AKZO NOBEL NV EUR0.50(POST REV SPLIT) -0.37% 11 AMBUB DC Equity Ambu A/S 1.41% 11 ALV GY Equity Allianz SE -0.79% 12 AMP IM Equity Amplifon SpA 0.45% 12 AMS SQ Equity Amadeus IT Hldg SA -1.78% 13 ANTO LN Equity Antofagasta Hldgs 1.62% 13 VNA GY Equity Deutsche Annington Immobilien -0.27% 14 ASM NA Equity ASM Intl 0.65% 14 ASSAB SS Equity Assa Abloy B -1.11% 15 ASML NA Equity ASML Holding NV 0.82% 15 ATCOA SS Equity Atlas Copco AB A -0.68% 16 ATO FP Equity AtoS 0.51% 16 ATCOB SS Equity Atlas Copco AB B -0.66% 17 AZN LN Equity AstraZeneca Plc 0.38% 17 AV/ LN Equity Aviva -0.07% -

Genmab Announces Antibody Development Collaboration with Lundbeck
GENMAB ANNOUNCES ANTIBODY DEVELOPMENT COLLABORATION WITH LUNDBECK Summary: Genmab and Lundbeck enter into an agreement to develop antibody therapeutics. Copenhagen, Denmark; October 13, 2010 – Genmab A/S (OMX: GEN) announced today an agreement to create and develop human antibody therapeutics for disorders of the central nervous system (CNS) with H. Lundbeck A/S. Genmab will create novel human antibodies to three targets identified by Lundbeck. Lundbeck will have access to Genmab’s antibody creation and development capabilities, including its state of the art, fully automated pre-clinical antibody screening and characterization capabilities and its proprietary stabilized IgG4 and UniBody therapeutic antibody platforms. Lundbeck will have an option to take selected antibodies into clinical development at its own cost and subject to the payment of milestones and single-digit royalties to Genmab upon successful development and commercialization. Genmab will have a similar option to take selected antibodies into clinical development for cancer indications at its own cost and subject to the payment of milestones and single-digit royalties to Lundbeck. Under the terms of the agreement, Genmab will receive an upfront payment of €7.5 million (approximately DKK 56 million). Lundbeck will fully fund the development of the antibodies. If all milestones in the agreement are achieved, the total value of the agreement to Genmab would be approximately €38 million (approximately DKK 283 million), plus single-digit royalties. “We are pleased to enter into this collaboration with Lundbeck, world experts in the development of CNS therapeutics. It gives Genmab the opportunity to leverage our antibody technology and expertise and to expand our pipeline into a new and exciting therapeutic area without assuming a financial obligation,” said Jan van de Winkel, Chief Executive Officer of Genmab. -

Delivering on Our Commitment
Delivering on Our Commitment 2020 Annual Report Genmab A/S CVR No. 21 02 38 84 Table of Management’s Financial Contents Review Statements Using Science to Turn Insights into Medicine Our Purpose To improve the lives of patients with cancer by creating and developing innovative and differentiated antibody products. It is our reason for being. 2 2020 Annual Report Table of Management’s Financial Contents Review Statements Table of Contents Management’s Review Financial Statements 5 About Genmab 22 Our Business 85 Financial Statements for the Genmab Group 7 Timeline 23 Research and Development 8 2020 at a Glance Capabilities 133 Financial Statements of the Parent Company 9 Progress Toward Our 2025 Vision 24 Antibody Discovery and Development 149 Directors’ and Management’s 10 Chair’s Statement 25 Product Pipeline Statement on the Annual Report 12 Letter from the CEO 52 Antibody Technologies 150 Independent Auditor’s Report 15 Market Overview 59 Risk Management 17 2020 Achievements 63 Financial Review 18 Consolidated Key Figures 69 Shareholders and Share Information 153 Glossary 19 2021 Outlook 154 Forward Looking Statement 19 Key 2021 Priorities 71 Environmental, Social, and 155 Contact Information 20 Business Model Governance 21 Our Strategy 72 Commitment to Building a Sustainable and Socially Responsible Biotech Our Vision 73 Corporate Social Responsibility and Sustainability Commitments By 2025, our own 75 Human Capital Management 76 Stakeholder Engagement product has transformed 78 Corporate Governance cancer treatment, 80 Board of Directors -
Remuneration in Danish Large Cap Companies Benchmarking Executive Management and Board Remuneration
Remuneration in Danish Large Cap Companies Benchmarking executive management and board remuneration 2013-2017 Brochure / report title goes here | Section title goes here Contents Introduction 3 Key findings 4 Current trends 5 Overview 13 Methodology 14 Total remuneration of executive directors 15 Base salary 20 Pension 23 Bonus 24 Long-term incentives (LTIs) 28 Board remuneration 38 Board demographics 40 How can Deloitte help? 44 Our contacts 45 Appendix 46 2 Remuneration in Danish Large Cap Companies | Introduction Introduction This report gives an overview of and insight into all report in accordance with International Financial remuneration of executive directors and boards of Reporting Standards (IFRS). Financial reporting on listed companies within the Danish Large-Cap Index1. remuneration of executives is more specifically In March 2018, the Danish Large-Cap Index comprised governed by IFRS 2 and the Danish Financial 39 companies, the names of which are listed in the Statements Act. Remuneration of executive directors appendix. These companies represent some of is required by IFRS to be disclosed on both fixed and the largest Danish companies from a wide range variable elements for executive management. Although of industries, from financial services to energy and reporting this level of detail for all individual members supplies. of management is not a requirement, it is however best practice guidance from the Committee on Corporate Over the year to March 2018, two new companies Governance2. Long-Term Incentive (LTI) programmes entered the Danish Large-Cap Index. In October 2017, must also be disclosed separately in their entirety, the professional cleaning product manufacturer Nilfisk which includes all participants, both executives and Holding entered the index through its IPO, while Alm. -

Summary: Genmab Provides an Update on the Potential Regulatory
REPORT PURSUANT TO SECTION 28a OF THE DANISH SECURITIES TRADING ACT Copenhagen, Denmark; March 11, 2011 – Pursuant to Section 28a of the Danish Securities Trading Act, Genmab A/S (OMX: GEN) shall make public information on transactions by managerial employees and their related parties involving Genmab shares and related instruments, as follows: Name Jan van de Winkel Reason Chief Executive Officer Issuer Genmab A/S ID code/ ISIN DK 0010272202 Description Shares Transaction Purchase Trading date March 11, 2011 Market NASDAQ OMX Copenhagen A/S Number 19,000 Market value DKK 1,022,599 About Genmab A/S Genmab is a leading international biotechnology company focused on developing fully human antibody therapeutics for the potential treatment of cancer. Genmab’s world class discovery and development teams are using cutting-edge technology to create and develop products to address unmet medical needs. Our primary goal is to improve the lives of patients who are in urgent need of new treatment options. For more information on Genmab’s products and technology, visit www.genmab.com. Contact: Helle Husted, Vice President, Investor Relations T: +45 33 44 77 30; M: +45 25 27 47 13; E: [email protected] This Stock Exchange Release contains forward looking statements. The words “believe”, “expect”, “anticipate”, “intend” and “plan” and similar expressions identify forward looking statements. Actual results or performance may differ materially from any future results or performance expressed or implied by such statements. The important factors that -

Proxy Vote Record
Company Name Ticker Country Meeting Date Proposal Text Vote Instruction A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Receive Report of Board A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Accept Financial Statements and Statutory Reports For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Approve Discharge of Management and Board For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Approve Allocation of Income and Dividends of DKK 150 Per Share For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Reelect Jim Hagemann Snabe as Director For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Reelect Ane Maersk Mc-Kinney Uggla as Director For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Reelect Robert Maersk Uggla as Director For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Reelect Jacob Andersen Sterling as Director For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Reelect Thomas Lindegaard Madsen as Director For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Ratify PricewaterhouseCoopers as Auditors For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Authorize Board to Declare Extraordinary Dividend For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Approve DKK 784.9 Million Reduction in Share Capital via Share Cancellation For A.P. Moller-Maersk A/S MAERSK.B Denmark 23-Mar-20 Approve Guidelines for Incentive-Based Compensation for Executive Management and Board Against A.P.