How to Do Clinical Trials with a Cancer Drug Partner Insights
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Conte Annual Report 2000 / 2001
Coloplast’s Mission Contents Throughout the world we wish to be perceived as depend- Coloplast in brief 2 able providers of consumable products and services. 5 years’ key figures and ratios 3 Our customers are health care professionals and users. Our primary concern is to improve the quality of life of Board and Group Management 4 individuals suffering from a disabling condition. Management report 5 We respond quickly to market needs to ensure the highest level of customer satisfaction. We strive to offer Financial review 11 preferred product ranges based on innovation, advanced technology and cost-effectiveness. Risk factors 15 Accounting policies 17 All employees must be recognised for their empathy with user needs and dependability in business relations. Profit and loss account 19 It is our ambition to attract and retain the best human Balance sheet 20 resources. Consolidated cash flow statement 22 As individuals and as an organisation we will act respon- Auditors’ report and approvals 23 sibly and be socially and environmentally conscious. Notes 24 We strive to be the best within our businesses, thereby achieving growth and value for the company, the em ploy- ees and shareholders. Stakeholder reporting Annual Report 2000 / 2001 – value creation and knowledge management 32 Customers 33 Employees 38 Society 41 Shareholders 43 Indicators for value drivers and value propositions 48 Artist of the year 50 Group addresses Executives Coloplast Mission Coloplast A/S Coloplast, Conveen, Comfeel, Holtedam 1 Amoena, Sween, Compeed, DK-3050 Humlebæk Assura, Easiflex, EasiCath, Coloplast’s Annual Report for 2000/01 has its own Tel. + 45 49 11 11 11 SpeediCath, Biatain, Contreet, microsite on our corporate website at the address: Fax + 45 49 11 15 55 and Prema are registered trade- E-mail: [email protected] marks of the Coloplast Group. -

Aktienyt Novozymes
Aktienyt Novozymes Hold Udfordrende markeder dæmper salgsvæksten Uændret Hold-anbefaling siden 20/04/2016 Vi fastholder vores Hold-anbefaling på Novozymes Dagens regnskab er lidt svagere, end vi havde ventet. Hård priskonkurrence i ’Bioenergi’ og økonomisk Analysedato: 10/08/2016 pressede kunder i ’Landbrug og Foder’ dæmper Novozymes’ salgsvækst. Pris- fastsættelsen af Novozymes-aktien afspejler efter vores vurdering Novozymes’ Aktuel kurs kl. 12:11: kortsigtede forretningsmuligheder. 295,00 DKK Salgsvæksten skuffer i årets 2. kvartal Omsætningen på 3.429 mio. kr. i 2. kvartal 2016 er 3,3% dårligere end vores estimat (-3,5% i Begivenhed: forhold til markedsforventningen). Resultatet af primær drift på 961 mio. kr. i 2. kvartal 2016 er 2,9% under vores estimat (-2,6% i forhold til markedsforventningen). I forhold til vores for- 2. kvartalsregnskab ventning er det specielt salget i divisionerne ’Bioenergi’ og ’Landbrug og Foder’, der viser en svagere end ventet udvikling i 2. kvartal. Novozymes justerer salgsprognosen for 2016 i lokal Seneste analyse: valuta. Selskabet venter nu en salgsvækst i lokal valuta på 2-4% i 2016 mod tidligere 3-5%. 05/08/16 – Vækst i ’Vaskemid- Novozymes forventer, at salgsvæksten i danske kroner forbliver uændret på 1-3% i 2016. ler’ – Tilbagegang i ’Bioenergi’ Salgsvæksten i ’Vaskemidler’ overgår vores forventning Den organiske salgsvækst i divisionen ’Vaskemidler’ på 4% i 2. kvartal er lidt bedre end vo- res estimat på 3%. Vi hæfter hos ved, at salget vokser i Asien drevet af øget penetration af Kalender: enzymer i flydende vaskemidler. Yderligere noterer vi os, at den positive salgsudvikling er 26/10/16 – 3. -

Genmab Announces Data to Be Presented at 2017 ASCO Annual Meeting
Genmab Announces Data to be Presented at 2017 ASCO Annual Meeting Media Release 8 abstracts on Genmab programs scheduled for presentation at ASCO Two daratumumab oral presentations and five daratumumab poster presentations Copenhagen, Denmark; April 20, 2017 – Genmab A/S (Nasdaq Copenhagen: GEN) announced today that seven daratumumab abstracts have been accepted for presentation at the 2017 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, June 2 – 6. These abstracts, submitted by our collaboration partner, Janssen Biotech, Inc., include updates for the POLLUX and CASTOR trials, and the first data for a Phase I study evaluating daratumumab with carfilzomib, lenalidomide and dexamethasone in front line multiple myeloma patients, which will be presented in an oral presentation. In addition, descriptions of the Phase Ib/II study of daratumumab plus atezolizumab in non-small cell lung cancer and of our Phase I/II study with HuMax-AXL-ADC are scheduled for poster presentations at the meeting. The titles of the abstracts are currently available on the ASCO website with the full abstracts scheduled to be published on May 17, 2017. “We are very pleased that, once again, a number of abstracts based on exciting work with Genmab’s innovative therapeutic antibody products have been accepted for presentation at the prestigious ASCO conference,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. List of abstracts: Daratumumab: Efficacy Of Daratumumab In Combination with Lenalidomide Plus Dexamethasone (DRd) or -

Genmab's 2020 Capital Markets
WELCOME Genmab’s 2020 Capital Markets Day November 13, 2020 Webcast Live from Utrecht and Princeton Forward Looking Statement This presentation contains forward looking statements. The words “believe”, “expect”, “anticipate”, “intend” and “plan” and similar expressions identify forward looking statements. All statements other than statements of historical facts included in this presentation, including, without limitation, those regarding our financial position, business strategy, plans and objectives of management for future operations (including development plans and objectives relating to our products), are forward looking statements. Such forward looking statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward looking statements. Such forward looking statements are based on numerous assumptions regarding our present and future business strategies and the environment in which we will operate in the future. The important factors that could cause our actual results, performance or achievements to differ materially from those in the forward looking statements include, among others, risks associated with product discovery and development, uncertainties related to the outcome of clinical trials, slower than expected rates of patient recruitment, unforeseen safety issues resulting from the administration of our products in patients, uncertainties related to product manufacturing, the lack of market acceptance of our products, our inability to manage growth, the competitive environment in relation to our business area and markets, our inability to attract and retain suitably qualified personnel, the unenforceability or lack of protection of our patents and proprietary rights, our relationships with affiliated entities, changes and developments in technology which may render our products obsolete, and other factors. -

Aktiekommentar Novozymes
Aktiekommentar Novozymes Sælg Novozymes står på dørtærsklen til et kommercielt Uændret gennembrud i 2G-biobrændstofindustrien Aktuel kurs: 216,30 DKK Begivenhed: Vi fastholder Sælg-anbefalingen på Novozymes. Værdiansættelsen af Novozy- mes-aktien indregner efter vores vurdering allerede store forventninger til Novo- zymes’ fremtidige salg og indtjening fra 2G-biobrændstofenzymer. 2G-industrien Optakt til kapitalmar- er dog i sin spæde opstartsfase. Der er fortsat stor usikkerhed forbundet med kedsdag hvilke teknologiske løsninger, der vil blive markedsledende. Vi forventer dog, at Novozymes vil få en betydelig markedsposition som leverandør til 2G-industrien. Baggrund: Novozymes afholder kapitalmarkedsdag den 31. oktober 2013. Det overordnende tema for dagen er anden generations biobrændstof (2G) og mulighederne for Novozymes i denne indu- stri. Verdens første kommercielle 2G-bioethanolproduktion er for nylig startet op i Cresentino i Italien med Novozymes som enzymleverandør. Produktionsanlægget er opført af italienske Beta Renewables, som Novozymes ejer 10% af. Konklusion: På kapitalmarkedsdagen vil vi have fokus på følgende punkter: - Produktionsøkonomien på det italienske 2G-anlæg – er det kommercielt konkurren- cedygtigt? - Statusopdatering på Novozymes’ globale partnerskabsaftaler – tidshorisont for kom- merciel 2G-produktion på forskellige markeder - Novozymes/Beta Renewables styrker i forhold til konkurrerende teknologier Vi forventer, at Novozymes i 2015 kan opnå et globalt enzymsalg til 2G- biobrændstofsproduktion på 213 mio. kr. stigende til 2.166 mio. kr. i 2020. Vi forventer, at Ki- na og Brasilien vil agere vækstmotor for udviklingen af 2G-industrien, mens vi først forventer, at udviklingen i USA kommer op i tempo efter 2015. Det er efter vores vurdering helt centralt, at Novozymes har indgået et samarbejde med itali- enske Beta Renewables. -

Second Pre-Clinical Milestone Met in Lundbeck Collaboration - €1 Million Milestone Payment to Genmab
GENMAB REACHES SECOND MILESTONE IN LUNDBECK COLLABORATION - Second pre-clinical milestone met in Lundbeck collaboration - €1 million milestone payment to Genmab Copenhagen, Denmark; February 10, 2012 – Genmab A/S (OMX: GEN) announced today it had reached the second pre-clinical milestone in the collaboration with H. Lundbeck A/S, triggering a €1 million payment. Genmab has reached the second milestone in the collaboration with H. Lundbeck A/S to create and develop human antibody therapeutics for disorders of the central nervous system (CNS). The milestone triggers a payment of €1 million to Genmab. Under the collaboration with Lundbeck Genmab creates novel human antibodies to three targets identified by Lundbeck and Lundbeck has access to Genmab’s antibody creation and development capabilities, including its state of the art, fully automated pre-clinical antibody screening and characterization capabilities and its proprietary stabilized IgG4 and UniBody therapeutic antibody platforms. Under the terms of the agreement, Genmab received an upfront payment of €7.5 million in October 2010 (approximately DKK 56 million). Lundbeck fully funds the development of the antibodies. If all milestones in the agreement are achieved, the total value of the agreement to Genmab would be approximately €38 million (approximately DKK 283 million), plus single-digit royalties. “We are very pleased to have met the in vitro proof of concept milestone for another target in the Lundbeck collaboration. This partnership is progressing well, with this second milestone coming shortly after we achieved the first preclinical milestone in December last year,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. -

Novozymes Continues to Deliver on the Global Goals
Novozymes continues to deliver on the Global Goals At the UN General Assembly in New York in September 2015, Novozymes joined the rest of the world in welcoming the UN Sustainable Development Goals (SDGs) and the 2030 Agenda for sustainable development. This agenda puts the world on a path toward a more sustainable, viable, low-carbon future, with the SDGs providing a partnership platform for governments, civil society and business to help tackle the world’s challenges. As a global market leader in biological solutions, producing a wide range of industrial enzymes and microorganisms, the intrinsically sustainable nature of Novozymes’ solutions enables us to contribute to one or more of the Sustainable Development Goals (SDGs), directly or indirectly. Every day, we strive to do our part to deliver on the SDGs, from the core of our business and right through our operations. Our contributions are outlined below. In addition, read ‘Working with the SDGs’ in ‘The Novozymes Report 2020’. Embracing our responsibility To shape a future of better business for us, our customers and the world, we need to have a sound, balanced and growing business. This will allow us to continue investing in our leadership position within biotechnology and capture new opportunities in the industries we serve. Our strategy Better business with biology is inspired by the societal needs defined by the Global Goals. Through the strategy, we are committed to developing solutions that can contribute to solving three global challenges: Climate, Water and Production and Consumption. We are also committed to operating our business responsibly and investing to minimize our negative environmental footprint. -

Interim Report for the First Quarter of 2020
Genmab Announces Financial Results for the First Quarter of 2020 May 6, 2020; Copenhagen, Denmark; Interim Report for the First Quarter Ended March 31, 2020 Highlights DARZALEX® (daratumumab) net sales increased approximately 49% compared to the first quarter of 2019 to USD 937 million, resulting in royalty income of DKK 775 million DARZALEX approved in Europe in combination with bortezomib, thalidomide and dexamethasone for the treatment of adult patients with newly diagnosed multiple myeloma who are eligible for autologous stem cell transplant U.S. FDA approved TEPEZZA™ (teprotumumab-trbw), developed and commercialized by Horizon Therapeutics, for thyroid eye disease U.S. FDA accepted, with priority review, Novartis’ supplemental Biologics License Application for subcutaneous ofatumumab in relapsing multiple sclerosis Anthony Pagano appointed Chief Financial Officer Anthony Mancini appointed Chief Operating Officer “Despite the unprecedented challenges posed by the coronavirus (COVID-19) pandemic, we will continue to invest in our innovative proprietary products, technologies and capabilities and use our world-class expertise in antibody drug development to create truly differentiated products with the potential to help cancer patients. While Genmab is closely monitoring the developments in the rapidly evolving landscape, we are extremely fortunate to have a solid financial foundation and a fabulous and committed team to carry us through these uncertain times,” said Jan van de Winkel, Ph.D., Chief Executive Officer of Genmab. Financial Performance First Quarter of 2020 Revenue was DKK 892 million in the first quarter of 2020 compared to DKK 591 million in the first quarter of 2019. The increase of DKK 301 million, or 51%, was mainly driven by higher DARZALEX royalties. -

Invitation Annual Shareholders' Meeting 2015
INVITATION ANNUAL SHAREHOLDERS’ MEETING 2015 To the shareholders of Novozymes A/S The Board of Directors is pleased to invite you to the Company’s Annual Shareholders’ Meeting on WEDNESDAY FEBRUARY 25, 2015 AT 4:00 P.M. at Ballerup Super Arena, Ballerup Idrætsby 4, 2750 Ballerup, Denmark AGENDA: 1. Report on the Company’s activities 5. Election of Chairman The Board of Directors proposes re-election of the 2. Presentation and approval of the audited incumbent Chairman. annual report Henrik Gürtler 3. Resolution on distribution of profit in Born 1953, Chairman, MSc accordance with the approved annual (Engineering), chemical engi- report neer. The Board of Directors re- The Board of Directors proposes a dividend of DKK commends re-election of 3.00 per A/B share of DKK 2 Henrik Gürtler because of his in-depth knowledge of Novozymes’ business, and expertise in managing and 4. Approval of remuneration to members of working in an international biotechnology company. the Board of the Directors for the present Henrik Gürtler serves as chairman of the board of Det year Alm. Danske Ejendomsselskab A/S (DADES A/S). Henrik Gürtler has been a member of the Board of The Board of Directors proposes that the fees for the Directors since 2000. board members and the Audit Committee shall be fixed to: 6. Election of Vice Chairman • The base fee for board members shall be DKK The Board of Directors proposes re-election of the 500,000 incumbent Vice Chairman. • The Chairman of the Board of Directors shall receive 3.0 times the base fee Agnete Raaschou-Nielsen • The Vice Chairman of the Board of Directors shall Born 1957, Chairman of receive 2.0 times the base fee Brdr. -

Aktienyt Novozymes
Aktienyt Novozymes Hold To cylindre i salgsvækstmotoren sætter ud Uændret Vi fastholder Hold-anbefalingen på Novozymes. Dagens regnskab byder på en Aktuel kurs: overraskende nedjustering af den organiske salgsvækst drevet af udfordringer inden for vaskemidler og bioenergi. Vi forventer ikke, at Novozymes vil være i 321,1 DKK stand til at løse udfordringerne på den korte bane. Begivenhed: Salgsvæksten skuffer markant i 2. kvartal Omsætningen på 3.449 mio. kr. i 2. kvartal 2015 er 5,2 % dårligere end vores estimat (-6,1% 2. kvartalsregnskab ift. markedsforventningerne). Resultatet af primær drift på 930 mio. kr. i 2. kvartal 2015 er 4,7% dårligere, end vi havde forventet (-6,3% ift. markedsforventningerne). Det skal dog bemærkes, at driftsresultatet i 2. kvartal er negativt påvirket af en nedskrivning på 50 mio. kr. Seneste analyse: relateret til Novozymes’ farmaceutiske forretning. Når der ses bort fra nedskrivningen er 17/04/15 – Aktieudsyn – Min- overskudsgraden i 2. kvartal på 28,4%, hvilket er markant over vores estimat på 26,8%. No- dre valuta-drevet nedjustering vozymes nedjusterer salgsprognosen for 2015. Novozymes forventer nu, at salget vil vokse på vej 13-16% i danske kroner i 2015 mod tidligere 16-18%. I lokal valuta forventer Novozymes, at salget vil vokse med 4-7% mod tidligere 7-9%. Den stærke udvikling i indtjeningen i årets første seks måneder betyder, at Novozymes fastholder indtjeningsprognosen for 2015 på trods af den svagere salgsudvikling. Kalender: 22/10/15 – 3. kvartalsregnskab Hård konkurrence bland nordamerikanske vaskemiddelproducenter skaber udfordringer 19/01/16 – Årsregnskab 2015 Omsætningen fra vaskemiddelenzymer er 2% under vores estimat. -
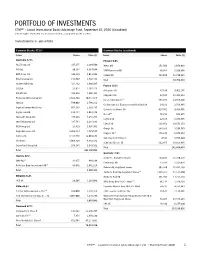
Portfolio of Investments
PORTFOLIO OF INVESTMENTS CTIVP® – Lazard International Equity Advantage Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.6% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 6.9% Finland 1.0% AGL Energy Ltd. 437,255 4,269,500 Metso OYJ 153,708 2,078,669 ASX Ltd. 80,181 4,687,834 UPM-Kymmene OYJ 36,364 1,106,808 BHP Group Ltd. 349,229 9,021,842 Valmet OYJ 469,080 11,570,861 Breville Group Ltd. 153,867 2,792,438 Total 14,756,338 Charter Hall Group 424,482 3,808,865 France 9.5% CSL Ltd. 21,611 4,464,114 Air Liquide SA 47,014 7,452,175 Data#3 Ltd. 392,648 1,866,463 Capgemini SE 88,945 11,411,232 Fortescue Metals Group Ltd. 2,622,808 30,812,817 Cie de Saint-Gobain(a) 595,105 24,927,266 IGO Ltd. 596,008 1,796,212 Cie Generale des Etablissements Michelin CSA 24,191 2,596,845 Ingenia Communities Group 665,283 2,191,435 Electricite de France SA 417,761 4,413,001 Kogan.com Ltd. 138,444 2,021,176 Elis SA(a) 76,713 968,415 Netwealth Group Ltd. 477,201 5,254,788 Legrand SA 22,398 1,783,985 Omni Bridgeway Ltd. 435,744 1,234,193 L’Oreal SA 119,452 38,873,153 REA Group Ltd. 23,810 1,895,961 Orange SA 298,281 3,106,763 Regis Resources Ltd. -

The Novozymes Report 2017
The Novozymes Report 2017 Rethink tomorrow Contents The big picture 4 2017 in brief Accounts and performance 15 Letter from the Board of Directors 66 Performance and consolidated financial statements 18 Letter from the CEO 74 Performance and environmental data 21 Novozymes in a nutshell 76 Performance and social & governance data 78 Notes 150 Statements Our business 156 Financial statements for Novozymes A/S 26 Business model 31 Strategy 33 Risk management 38 Targets Governance 49 Corporate governance 54 Board of Directors 57 Executive Leadership Team 59 Remuneration report 63 The Novozymes stock Novozymes A/S 2 The big picture Financial highlights DKK 4% 27.9% Organic sales growth EBIT margin Sales grew by 4% organically and by 3% in DKK. EBIT margin at 27.9% was on par with last year. Sales to Food & Beverages and Bioenergy were Excluding the one-time costs relating to layoffs the most significant contributors to organic in 2017, the cost associated with the change Key figures 2017 2017 sales growth in 2017. to the Executive Leadership Team, and the loss realized outlook* relating to the Albumedix divestment, the EBIT margin was ~29% Sales growth, organic 4% 2-5% Sales growth, DKK 3% 3-6% EBIT growth 3% 3-6% EBIT margin 27.9% ~28% Net profit growth 2% 2-5% Net investments excl. 1,665 1,700- acquisitions, DKKm 1,900 Free cash flow before 2,398 2,000- acquisitions, DKKm 2,200 2% 25.6% ROIC (including goodwill) 25.6% 24-25% Net profit growth ROIC * Outlook guided as of January 18, 2017.