Ross Procedure Vs Mechanical Aortic Valve Replacement in Adults a Systematic Review and Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
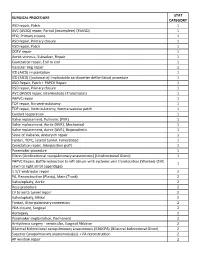
Surgeries by STAT Category
STAT SURGICAL PROCEDURE CATEGORY ASD repair, Patch 1 AVC (AVSD) repair, Partial (Incomplete) (PAVSD) 1 PFO, Primary closure 1 ASD repair, Primary closure 1 VSD repair, Patch 1 DCRV repair 1 Aortic stenosis, Subvalvar, Repair 1 Coarctation repair, End to end 1 Vascular ring repair 1 ICD (AICD) implantation 1 ICD (AICD) ([automatic] implantable cardioverter deFibrillator) procedure 1 ASD Repair, Patch + PAPCV Repair 1 VSD repair, Primary closure 1 AVC (AVSD) repair, Intermediate (Transitional) 1 PAPVC repair 1 TOF repair, No ventriculotomy 1 TOF repair, Ventriculotomy, Nontransanular patch 1 Conduit reoperation 1 Valve replacement, Pulmonic (PVR) 1 Valve replacement, Aortic (AVR), Mechanical 1 Valve replacement, Aortic (AVR), Bioprosthetic 1 Sinus oF Valsalva, Aneurysm repair 1 Fontan, TCPC, Lateral tunnel, Fenestrated 1 Coarctation repair, Interposition graFt 1 Pacemaker procedure 1 Glenn (Unidirectional cavopulmonary anastomosis) (Unidirectional Glenn) 1 PAPVC Repair, BaFFle redirection to leFt atrium with systemic vein translocation (Warden) (SVC 1 sewn to right atrial appendage) 1 1/2 ventricular repair 2 PA, Reconstruction (Plasty), Main (Trunk) 2 Valvuloplasty, Aortic 2 Ross procedure 2 LV to aorta tunnel repair 2 Valvuloplasty, Mitral 2 Fontan, Atrio-pulmonary connection 2 PDA closure, Surgical 2 Aortopexy 2 Pacemaker implantation, Permanent 2 Arrhythmia surgery - ventricular, Surgical Ablation 2 Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (Bilateral bidirectional Glenn) 2 Superior Cavopulmonary anastomosis(es) + PA -

Appendix A: Surgical Procedure Terms and Definitions
Appendix A: Surgical Procedure Terms and Definitions Anomalous Systemic Venous Connection Anomalous Systemic Venous Connection Repair Repair includes a range of surgical approaches, including, among others: ligation of anomalous vessels, reimplantation of anomalous vessels (with or without use of a conduit), or redirection of anomalous systemic venous flow through directly to the pulmonary circulation (bidirectional Glenn to redirect LSVC or RSVC to left or right pulmonary artery, respectively). Aortic Aneurysm Aortic aneurysm repair Aortic aneurysm repair by any technique. Aortic Dissection Aortic Dissection repair Aortic dissection repair by any technique. Aortic Root Replacement Aortic Root Replacement, Bioprosthetic Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a bioprosthesis (e.g., porcine) in a conduit, often composite. Aortic Root Replacement, Mechanical Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a mechanical prosthesis in a composite conduit. Aortic Root Replacement, Homograft Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) with a homograft Aortic Root Replacement, Valve sparing Replacement of the aortic root (that portion of the aorta attached to the heart; it gives rise to the coronary arteries) without replacing the aortic valve (using a tube graft). Aortic Valve Disease Ross Procedure Replacement of the aortic valve with a pulmonary autograft and replacement of the pulmonary valve with a homograft conduit. Konno Procedure (with and without aortic valve replacement) Relief of left ventricular outflow tract obstruction associated with aortic annular hypoplasia, aortic valvar stenosis and/or aortic valvar insufficiency via Konno aortoventriculoplasty. -

The Ross Inclusion Dacron Graft
551 Masters of Cardiothoracic Surgery The Ross inclusion Dacron graft Jama Jahanyar, Daniel E. Muñoz, Saadallah Tamer, Gebrine El Khoury, Laurent de Kerchove Department of Cardiovascular & Thoracic Surgery, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain (UCL), Brussels, Belgium Correspondence to: Laurent de Kerchove, MD, PhD. Department of Cardiovascular & Thoracic Surgery, Cliniques Universitaires Saint-Luc, Avenue Hippocrate 10, 1200 Brussels, Belgium. Email: [email protected]. Submitted Aug 04, 2020. Accepted for publication May 05, 2021. doi: 10.21037/acs-2020-rp-17 View this article at: http://dx.doi.org/10.21037/acs-2020-rp-17 Clinical vignette The tip of a hemostat grabs the right ventricular muscle in the lower triangle of the anterior pulmonary commissure. We present the case of a twenty-eight-year-old male with The hemostat tip is then pushed through the anterior severe aortic stenosis and mean gradient across the aortic wall of the right ventricular outflow tract (RVOT), and valve (AV) of 52 mmHg, due to a calcified congenital countertraction is provided by the surgeon’s hand. Starting unicuspid AV. The patient was asymptomatic, without at the point of RVOT muscle penetration, the pulmonary significant past medical history, and clean coronaries. autograft is excised circumferentially, 3–4 mm proximal Due to extensive AV calcifications, AV repair was not to the pulmonary valve, avoiding injury to the first septal feasible, and Ross procedure was indicated. Echocardiography branch of the left anterior descending artery. Cardioplegia identified a dilated aortic annulus of 28 mm; the aortic is given to identify septal bleeders and hemostasis achieved. -

NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures
NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures Measure Number: PCS‐001‐09 Measure Title: Participation in a national database for pediatric and congenital heart surgery Description: Participation in at least one multi‐center, standardized data collection and feedback program that provides benchmarking of the physician’s data relative to national and regional programs and uses process and outcome measures. Participation is defined as submission of all congenital and pediatric operations performed to the database. Numerator Statement: Whether or not there is participation in at least one multi‐center data collection and feedback program. Denominator Statement: N/A Level of Analysis: Group of clinicians, Facility, Integrated delivery system, health plan, community/population Data Source: Electronic Health/Medical Record, Electronic Clinical Database (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Clinical Registry (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Claims, Paper Medical Record Measure Developer: Society of Thoracic Surgeons Type of Endorsement: Recommended for Time‐Limited Endorsement (Steering Committee Vote, Yes‐9, No‐0, Abstain‐0) Attachments: “STS Attachment: STS Procedure Code Definitions” Meas# / Title/ Steering Committee Evaluation and Recommendation (Owner) PCS-001-09 Recommendation: Time-Limited Endorsement Yes-9; No-0; Abstain-0 Participatio n in a Final Measure Evaluation Ratings: I: Y-9; N-0 S: H-4; M-4; L-1 U: H-9; M-0; L-0 national F: H-7; M-2; L-0 database for Discussion: pediatric I: The Steering Committee agreed that this measure is important to measure and report. and By reporting through a database, it is possible to identify potential quality issues and congenital provide benchmarks. -

Distribution of Heart Disease in a Multicentre Paediatric Cardiac Registry
TABLE 1: Geometrical comparison Volume 10 • Number 1 LT (n=7) EC (n=3) Summer 2013 T1 T2 Normalised Change T1 T2 Normalised Change Body surface area (m2) 0.9±0.2 1.4±0.2 0.8±0.1 1.4±0.3 Age at CMR (years) 8.7±3.4 13.5±1.9 8.7±2.1 12.7±1.5 Mean diameter (mm) FP 14.9±3.8 18.4±4.7 5.4±2.1 15.1±0.3 15.8±0.6 0.8±0.7 SVC 11.7±3.4 15.0±5.4 5.2±5.3 12.9±0.2 14.5±1.7 2.0±1.3 dAo 10.3±1.5 13.6±1.8 5.2±2.0 11.7±0.5 11.8±1.0 0.3±0.9 LPA 10.6±3.0 11.7±3.0 1.6±3.6 10.3±2.1 11.2±5.2 0.3±4.8 RPA 10.9±3.9 14.4±3.3 5.2±3.0 11.1±2.8 11.3±4.4 -0.3±2.2 Diameter at PA branching (mm) 16.4±5.7 18.9±9.0 3.8±6.2 15.6±1.7 15.1±1.8 -0.6±0.1 FP volume (cm3) 16.1±7.6 29.4±15.7 20.9±14.8 13.0±1.9 16.8±3.0 5.4±1.1 Mean Nakata index (mm2/m2) 207.5±52.6 205.2±45.4 237.7±99.4 154.4±60.6 Mean McGoon ration 2.1±0.4 1.9±0.3 1.8±0.3 1.9±0.7 TABLE 2: Haemodynamic metrics for the LT group Cardiac index (L/min/m2) T1 2.8±0.6 T2 2.8±0.4 TCPC resistance (WU) S1 (T1 geometry, T1 fl ow) 0.5±0.3 S2 (T2 geometry, T2 fl ow) 0.5±0.4 S3 (T1 geometry, T2 fl ow) 0.7±0.8 Serial increase (S2 - S1) 20% Growth absence (S3 - S1) 57% Results are shown as mean ± standard deviation. -

Reinforced Ross Operation and Intermediate to Long Term Follow Up
1223 Review Article on Management of Congenital Heart Disease Reinforced Ross operation and intermediate to long term follow up Awais Ashfaq, Hayden Leeds, Irving Shen, Ashok Muralidaran Section of Pediatric and Congenital Cardiac Surgery, Division of Cardiothoracic Surgery, Department of Surgery, Doernbecher Children’s Hospital, Oregon Health & Science University, Portland, OR, USA Contributions: (I) Conception and design: A Muralidaran, I Shen; (II) Administrative support: A Muralidaran, I Shen; (III) Provision of study materials or patients: A Muralidaran, I Shen; (IV) Collection and assembly of data: A Ashfaq, H Leeds; (V) Data analysis and interpretation: A Ashfaq, H Leeds; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Ashok Muralidaran, MD. Associate Professor of Surgery and Pediatrics, Pediatric and Congenital Cardiac Surgery, Doernbecher Childrens Hospital, Oregon Health & Sciences University, Portland, OR, USA. Email: [email protected]. Abstract: The Ross operation for aortic valve replacement continues to be a controversial option because of concerns related to late autograft dilation and progressive neo-aortic regurgitation. We described a technique in 2005 to address this problem, in which we place the entire autograft in a Dacron tube which makes it theoretically unlikely, if not impossible, for it to dilate—the reinforced Ross procedure. Since 2004, we have operated on 25 patients using this technique. Median length of follow-up in our cohort was 6 years, with 14 patients having 5 years or more of follow-up. Our data demonstrate the externally supported, or reinforced Ross technique using a straight graft is a safe and effective technique in older children, adolescents, and young adult patients. -

82064907.Pdf
Brief Communications Successful removal of migrated acupuncture needles in a patient with cardiac tamponade by means of intraoperative transesophageal echocardiographic assistance Jae-Hyeong Park, MD,a Hong Ju Shin, MD,b Suk Jung Choo, MD,b Jae Kwan Song, MD,c and Jae-Joong Kim, MD,c Jeonju and Seoul, Korea ardiac tamponade is a potentially life-threatening con- The emergency cardiac operation was done to remove the dition that can cause death if not diagnosed and treated foreign bodies with intraoperative transesophageal echocardio- promptly. Here we describe a case of cardiac tampon- graphic guidance (Sonos 2000; Hewlett-Packard Co, Andover, ade caused by penetration of the right ventricular free Mass) with a multiplane probe. The transesophageal echocardio- Cwall by migrated acupuncture needles. The needles were detected gram showed a needle-shaped mass crossing the interventricular and successfully removed with intraoperative transesophageal septum. After the pericardiectomy, the needle-shaped foreign ma- echocardiographic assistance. terial was seen penetrating the right ventricular wall and was removed successfully. Despite of the removal, an intraoperative Clinical Summary transesophageal echocardiography revealed another needle-shaped A 49-year-old woman was admitted to our hospital after having foreign body crossing from the interventricular septum to the left chest pain and was then hospitalized 2 hours later after syncope. ventricular cavity (Figure 2, A). After a cardiopulmonary bypass, She had a history of recurrent episodes of pulmonary thromboem- her left atrium was opened, and the foreign body was successfully bolism that arose from deep vein thrombosis. A Bird’s Nest filter removed. The removed foreign bodies were thin, needle-shaped (Cook Co, Leechburg, Pa) was implanted in her inferior vena cava materials (Figure 2, B). -

Ross Procedure for Heart Valve Disease Your Healthcare Team May Have Discussed the Need for Heart Valve If You Have Surgery with You
Northwestern Memorial Hospital Patient Education Bluhm Cardiovascular Institute ABOUT YOUR SURGERY Cardiac Surgery: Ross Procedure for Heart Valve Disease Your healthcare team may have discussed the need for heart valve If you have surgery with you. To better understand these discussions and what to expect, this brochure will explain how the heart valves any questions, work and the types of valve disease. You will learn about the Ross procedure for valve disease. please ask Understanding valve disease your physician To understand heart valve disease, it is helpful to understand how the valves in the heart work. There are 4 heart valves: mitral, or nurse. tricuspid, aortic and pulmonary. Valves consist of either 2 or 3 leaflets. Each valve opens and closes about 50 million times a year, up to 4 billion times in a typical lifetime! The valves keep the blood moving forward through the heart and out to the rest of the body. In a front view of the heart, when the mitral valve on the left side of the heart and the tricuspid valve on the right side of the heart open (Figure 1), blood flows into the left and right ventricles (lower heart chambers). The aortic and pulmonic valves are closed so the ventricles can fill with blood. Figure 1. Closed aortic and pulmonary valves Aortic valve Mitral valve Pulmonary valve Tricuspid valve Copyright ©2015 Krames Staywell LLC Figure 2. Open aortic and pulmonary valves When the mitral and tricuspid valves close, the aortic and pulmonic valves open (Figure 2). When the pulmonary valve opens, blood is pumped into Mitral valve the blood vessels of the lungs. -

Surgical Data Collection Form AVSD Other Surgical Procedure Form
AVSD – A CHSS Inception Cohort Study: Surgical Data Collection Form Study Number ☐☐☐☐☐☐☐☐ Event Number ☐☐ Operation Date (DD/MMM/YYYY) ☐☐/☐☐☐/☐☐☐☐ Procedure ☐PA Band ☐Norwood Procedure ☐Glenn Procedure ☐Hemi-Fontan ☐Fontan Procedure ☐One and Half Ventricle Repair ☐Heart Transplant ☐Subsequent AVSD Procedures ☐Hybrid Procedure ☐Interval Procedure If Fontan, specify type: ☐ECC ☐Lateral Tunnel ☐Transcatheter If Subsequent AVSD Procedure, specify type: ☐Atrial Fenestration ☐Ventricular Fenestration ☐AVSD Repair Takedown ☐PA Plasty ☐LAVV Repair ☐LAVV Replacement ☐RAVV Repair ☐RAVV Replacement ☐CAVV Repair ☐CAVV Replacement ☐ASD Repair ☐VSD Repair Concomitant Procedure 1: ☐Damus-Kaye-Stansel procedure (DKS) (creation of AP anastomosis without arch reconstruction) ☐Cavopulmonary anastomosis Bidirectional (BDCPA) (bidirectional Glenn) ☐Cavopulmonary Glenn (unidirectional cavopulmonary anastomosis) (unidirectional Glenn) ☐Cavopulmonary Bilateral bidirectional cavopulmonary anastomosis (BBDCPA) (bilateral bidirectional Glenn) ☐HemiFontan ☐Cavopulmonary anastomosis(es) (Glenn or HemiFontan) + Atrioventricular valvuloplasty ☐Cavopulmonary anastomosis(es) + PA reconstruction (Superior) ☐Palliation, Other (if other explain) ☐ECMO cannulation ☐ECMO decannulation ☐ECMO procedure AVSD Other Surgical Procedure Form Version Date: February 17, 2016 Page 1 of 26 AVSD – A CHSS Inception Cohort Study: Surgical Data Collection Form ☐Intraaortic balloon pump (IABP) insertion ☐Right/left heart assist device procedure ☐VAD explantation ☐VAD implantation ☐Echocardiography -

Cardiology in the Young
Volume 25 Supplement 1 Pages S1–S180 doi:10.1017/S1047951115000529 Cardiology in the Young © 2015 Cambridge University Press 49th Annual Meeting of the Association for European Paediatric and Congenital Cardiology, AEPC with joint sessions with the Japanese Society of Pediatric Cardiology and Cardiac Surgery, Asia-Pacific Pediatric Cardiology Society, European Association for Cardio-Thoracic Surgery and Canadian Pediatric Cardiology Association, Prague, Czech Republic, 20–23 May 2015 YIA-1 lesions and significantly decreased an anti-apoptotic molecule Macitentan Reverses Early Obstructive Pulmonary survivin protein and mRNA expression in lungs and the propor- Vasculopathy in Rats: Early Intervention in Overcoming tion of survivin positive αSMA cells in occlusive lesions in the early the Survivin-mediated Resistance to Apoptosis study but not in the late study. Shinohara T. (1,4), Sawada H. (1), Otsuki S. (1), Yodoya N. (1), Conclusions: Macitentan reversed early but not late obstructive pul- Kato T. (1), Ohashi H. (1), Zhang E. (2), Saitoh S. (4), monary vascular disease in rats. This reversal was associated with the Shimpo H. (3), Maruyama K. (2), Komada Y. (1), Mitani Y. (1) suppression of survivin-related resistance to apoptosis and prolifera- Department of Pediatrics (1); Anesthesiology and Critical Care Medicine tion of αSMA cells in occlusive lesions. These findings could be (2); Thoracic and Cardiovascular Surgery (3); Mie University Graduate mechanistic basis for the efficacy of early treatment and give an insight School of Medicine, Tsu, Japan, Department of Pediatrics and into later appearance of resistance to treatment for this disorder. Neonatology, Nagoya City University Graduate School of Medical Sciences, Nogoya, Japan (4) YIA-2 Objectives: We tested the hypothesis that a novel endothelin Does reversal of flow in the fetal aortic arch in second trimester receptor antagonist macitentan reverses the early and/or late stages aortic stenosis predict hypoplastic left heart syndrome? of occlusive pulmonary vascular disease in rats. -
W9646-Front Matter W9646-Front Matter.Qxd
W9646-Front matterISBN_W9646-Front matter.qxd 12/27/2011 7:24 PM Page i Understanding Pediatric Heart Sounds W9646-Front matterISBN_W9646-Front matter.qxd 12/27/2011 7:24 PM Page ii W9646-Front matterISBN_W9646-Front matter.qxd 12/27/2011 7:24 PM Page iii Understanding Pediatric Heart Sounds second edition Steven Lehrer, MD The Mount Sinai School of Medicine New York, New York Saunders An Imprint of Elsevier Science Philadelphia London New York St. Louis Sydney Toronto W9646-Front matterISBN_W9646-Front matter.qxd 12/27/2011 7:24 PM Page iv SAUNDERS An Imprint of Elsevier Science 11830 Westline Industrial Drive St. Louis, Missouri 63146 Understanding Pediatric Heart Sounds ISBN 1468138030 Copyright © 2003, Elsevier Science (USA). All rights reserved. 9781468138030 No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without prior permission of the publisher. NOTICE Health care is an ever-changing field. Standard safety precautions must be followed, but as new research and clinical experience broaden our knowledge, changes in treatment and drug therapy may become necessary or appropriate. Readers are advised to check the most current product information provided by the manufacturer of each drug to be administered to verify the recommended dose, the method and duration of administration, and contraindications. It is the responsibility of the licensed health care provider, relying on experience and knowledge of the patient, to determine dosages and the best treatment for each individual patient. Neither the publisher nor the editor assumes any liability for any injury and/or damage to persons or property arising from this publication. -
Resurgence of the Ross Procedure
514 Editorial Resurgence of the Ross procedure Robert O. Bonow Bluhm Cardiovascular Institute, Northwestern Memorial Hospital, Northwestern University Feinberg School of Medicine, Chicago, IL, USA Correspondence to: Robert O. Bonow, MD, MS. Department of Medicine, Northwestern University Feinberg School of Medicine, 676 North St. Clair Street, Suite 600, Chicago, IL 60611, USA. Email: [email protected]. Submitted Sep 10, 2020. Accepted for publication Dec 23, 2020. doi: 10.21037/acs-2020-rp-196 View this article at: http://dx.doi.org/10.21037/acs-2020-rp-196 Innovation in transcatheter aortic valve replacement and cardiac surgeons shy away from recommending (TAVR), which has virtually transformed the care of bioprosthetic heterografts and homografts for young adults patients with aortic stenosis (AS), has fueled an intense in the 20–40 years age range due to accelerated structural interest in the management of patients with aortic valve valve deterioration, the long-term consequences of life- disease. Indications for TAVR have expanded to low- long anticoagulant therapy with a vitamin K antagonist in risk symptomatic patients with AS, and clinical trials are patients with mechanical prostheses is equally daunting. currently in progress for asymptomatic patients with severe These same issues carry over to patients in ages 40–60 years. AS, testing the strategy of pre-emptive TAVR versus There is no perfect valve substitute for young and middle- waiting for symptom onset or other indications for valve aged patients with aortic valve disease. Against this replacement. continuing conundrum, the time is ripe for discussions on However, expanding the eligibility for TAVR to broadening the use of the Ross operation as a preferred lower risk, less symptomatic patients goes against the option for selected young individuals who require AVR (2).